Abstract
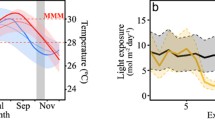
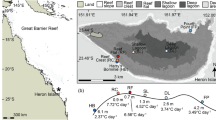
Reef-forming corals are under threat globally from climate change, leading to changes in sea temperatures with both hot and cold events recorded and projected to increase in frequency and severity in the future. Tolerance to heat and cold exposure has been found to be mutually exclusive in other marine invertebrates, but it is currently unclear whether a trade-off exists between hot and cold thermal tolerance in tropical corals. This study quantified the changes in physiology in Acropora millepora from the central Great Barrier Reef subjected to three temperature treatments; sub-lethal cold, ambient and sub-lethal heat (23.0 °C, 27.0 °C and 29.5 °C, respectively). After 10 weeks, pigment content and Symbiodiniaceae density increased in cold-treated corals but decreased in heat-treated corals relative to corals at ambient conditions. Heat-treated corals gained less mass relative to both ambient and cold-treated corals. These results indicate that the physiological condition of A. millepora corals examined here improved in response to mild cold exposure compared to ambient exposure and decreased under mild heat exposure despite both these temperatures occurring in situ around 15% of the year. The energetic condition of corals in the hotter treatment was reduced compared to both ambient and cooler groups, indicating that corals may be more resilient to mild cold exposure relative to mild heat exposure. The results indicate that the corals shifted their resource allocation in response to temperature treatment, investing more energy into skeletal extension rather than maintenance. No evidence of thermal tolerance trade-offs was found, and cold thermal tolerance was not lost in more heat-tolerant individuals. An enhanced understanding of physiological responses of corals at both ends of the thermal spectrum is important for predicting the resilience of corals under projected climate change conditions.


Similar content being viewed by others
References
Abrego D, Ulstrup KE, Willis BL, van Oppen MJ (2008) Species-specific interactions between algal endosymbionts and coral hosts define their bleaching response to heat and light stress. Proc R Soc B Biol Sci 275:2273–2282
Ainsworth TD, Heron SF, Ortiz JC, Mumby PJ, Grech A, Ogawa D, Eakin CM, Leggat W (2016) Climate change disables coral bleaching protection on the Great Barrier Reef. Science 352:338–342
Anderson AR, Collinge JE, Hoffmann AA, Kellett M, McKechnie SW (2003) Thermal tolerance trade-offs associated with the right arm of chromosome 3 and marked by the hsr-omega gene in Drosophila melanogaster. Heredity (Edinb) 90:195–202
Anthony KRN, Connolly SR, Willis BL (2002) Comparative analysis of energy allocation to tissue and skeletal growth in corals. Limnology 47:1417–1429
Baird AH, Marshall PA (2002) Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar Ecol Prog Ser 237:133–141
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci 80:435–471
Bay RA, Palumbi SR (2015) Rapid acclimation ability mediated by transcriptome changes in reef-building corals. Genome Biol Evol 7:1602–1612
Berkelmans R, van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a “nugget of hope” for coral reefs in an era of climate change. Proc Biol Sci 273:2305–2312
Berkelmans R, Willis BL (1999) Seasonal and local spatial patterns in the upper thermal limits of corals on the inshore Central Great Barrier Reef. Coral Reefs 18:219–228
Cai W, Wang G, Santoso A, McPhaden MJ, Wu L, Jin F-F, Timmermann A, Collins M, Vecchi G, Lengaigne M, England MH, Dommenget D, Takahashi K, Guilyardi E (2015) Increased frequency of extreme La Niña events under greenhouse warming. Nat Clim Chang 5:132–137
Clarke A (2003) Costs and consequences of evolutionary temperature adaptation. Trends Ecol Evol 18:573–581
Davies PS (1989) Short-tern growth measurements of corals using an accurate buoyant weighing technique. Mar Biol 101:389–395
De’ath G, Fabricius KE, Sweatman H, Puotinen M (2012) The 27 year-decline of coral cover on the Great Barrier Reef and its causes. PNAS 109:17995–17999
Dove SG, Kline DI, Pantos O, Angly FE, Tyson GW, Hoegh-Guldberg O (2013) Future reef decalcification under a business-as-usual CO2 emission scenario. PNAS 110:15342–15347
Eakin CM, Lough JM, Heron SF (2009) Climate variability and change: Monitoring data and evidence for increased coral bleaching stress. In: van Oppen MJH, Lough JM (eds) Coral Bleaching. Patterns, Processes, Causes and Consequences. Springer, Berlin, pp 41–67
Fisher PL, Malme MK, Dove S (2012) The effect of temperature stress on coral-Symbiodinium associations containing distinct symbiont types. Coral Reefs 31:473–485
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chemsitry 226:497–509
Gates RD, Baghdasarian G, Muscatine L (1992) Temperature stress causes host-cell detachment in symbiotic Cnidarians: implications for coral bleaching. Biol Bull 182:324–332
Gillooly JF, Brown JH, West GB, Savage VM, Charnow EL (2001) Effects of Size and Temperature on Metabolic Rate. Science 293:2248–2251
Higuchi T, Agostini S, Casareto BE, Suzuki Y, Yuyama I (2015) The northern limit of corals of the genus Acropora in temperate zones is determined by their resilience to cold bleaching. Sci Rep 5:18467
Hoegh-Guldberg O, Smith GJ (1989) The effect of sudden changes in temperature, light and salinity on the population density and export of zooxanthallae from the reef corals Stylophora pistillata (Esper) and Seriatopora hystrix (Dana). Jounral Exp Mar Biol Ecol 129:279–303
Howells EJ, Beltran VH, Larsen NW, Bay LK, Willis BL, van Oppen MJH (2011) Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat Clim Chang 2:116–120
Howells EJ, Berkelmans R, Van Oppen MJH, Willis BL, Bay LK (2013) Historical thermal regimes define limits to coral acclimatization. Ecology 94:1078–1088
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Butler IR, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin CM, Figueira WF, Gilmour JP, Harrison HB, Heron SF, Hoey AS, Hobbs J-PA, Hoogenboom MO, Kennedy EV, Kuo C-Y, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm HA, McWilliam MJ, Pandolfi JM, Pears RJ, Pratchett MS, Schoepf V, Simpson T, Skirving WJ, Sommer B, Torda G, Wachenfeld DR, Willis BL, Wilson SK (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Jokiel PL, Coles SL (1977) Effects of Temperature on the Mortality and Growth of Hawaiian Reef Corals. Mar Biol 43:201–208
Kemp DW, Oakley CA, Thornhill DJ, Newcomb LA, Schmidt GW, Fitt WK (2011) Catastrophic mortality on inshore coral reefs of the Florida Keys due to severe low-temperature stress. Glob Chang Biol 17:3468–3477
Krediet CJ, DeNofrio JC, Caruso C, Burriesci MS, Cella K, Pringle JR (2015) Rapid, Precise, and Accurate Counts of Symbiodinium Cells Using the Guava Flow Cytometer, and a Comparison to Other Methods. PLoS One 10:e0135725
Krueger T, Hawkins TD, Becker S, Pontasch S, Dove S, Hoegh-Guldberg O, Leggat W, Fisher PL, Davy SK (2015) Differential coral bleaching - contrasting the activity and response of enzymatic antioxidants in symbiotic partners under thermal stress. Comp Biochem Physiol A Mol Integr Physiol 190:15–25
Leuzinger S, Willis BL, Anthony KRN (2012) Energy allocation in a reef coral under varying resource availability. Mar Biol 159:177–186
Lirman D, Schopmeyer S, Manzello D, Gramer LJ, Precht WF, Muller-Karger F, Banks K, Barnes B, Bartels E, Bourque A, Byrne J, Donahue S, Duquesnel J, Fisher L, Gilliam D, Hendee J, Johnson M, Maxwell K, McDevitt E, Monty J, Rueda D, Ruzicka R, Thanner S (2011) Severe 2010 cold-water event caused unprecedented mortality to corals of the Florida reef tract and reversed previous survivorship patterns. PLoS One 6. https://doi.org/10.1371/journal.pone.0023047
Little AF, van Oppen MJH, Willis BL (2004) Flexibility in Algal Endosymbioses Shapes Growth in Reef Corals. Science 304:1492–1495
Lohr KE, Patterson JT (2017) Intraspecific variation in phenotype among nursery-reared staghorn coral Acropora cervicornis (Lamarck, 1816). J Exp Mar Bio Ecol 486:87–92
Madin JS, Hoogenboom MO, Connolly SR, Darlin ES, Falster DS, Huang D, Keith SA, Mizerek T, Pandolfi JM, Putnam HM, Baird AH (2016) A trait-based approach to advance coral reef science. Trends Ecol Evol 31:419–428
Mieog JC, Olsen JL, Berkelmans R, Bleuler-Martinez SA, Willis BL, van Oppen MJH (2009) The roles and interactions of symbiont, host and environment in defining coral fitness. PLoS One 4:e6364. https://doi.org/10.1371/journal.pone.0006364
Naumann MS, Niggl W, Laforsch C, Glaser C, Wild C (2009) Coral surface area quantification-evaluation of established techniques by comparison with computer tomography. Coral Reefs 28:109–117
Oliver TA, Palumbi SR (2011) Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30:429–440
Paz-García DA, Balart EF, García-de-Léon FJ (2012) Cold water bleaching of Pocillopora in the Gulf of California. Proc 12th Int Coral Reef Symp 9–13
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2017) nlme: Linear and Nonlinear Mixed Effects Models
Pontasch S, Fisher PL, Krueger T, Dove S, Hoegh-Guldberg O, Leggat W, Davy SK (2016) Photoacclimatory and photoprotective responses to cold. J Phycol. https://doi.org/10.1111/jpy.12492-15-123
Pörtner HO (2002) Climate variations and the physiological basis of temperature dependent biogeography: Systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol - A Mol Integr Physiol 132:739–761
Pratchett MS, Anderson KD, Hoogenboom MO, Widman E, Baird AH, Pandolfi JM, Edmunds PJ, Lough JM (2015) Spatial, temporal and taxonomic variation in coral growth - implications for the structure and function of coral reef ecosystems. Oceanogr Mar Biol An Annu Rev 53:215–296
R Core Team (2017) R: A language and environment for statistical computing
Ritchie RJ (2006) Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89:27–41
Rocker MM, Francis D, Fabricius KE, Willis BL, Bay LK (2017) Variation in the health and biochemical condition of the coral Acropora tenuis along two water quality gradients on the Great Barrier Reef. Mar Pollut Bull, Australia. https://doi.org/10.1016/j.marpolbul.2017.03.066
Rodolfo-Metalpa R, Peirano A, Houlbrèque F, Abbate M, Ferrier-Pagès C (2008) Effects of temperature, light and heterotrophy on the growth rate and budding of the temperate coral Cladocora caespitosa. Coral Reefs 27:17–25
Rodríguez-Troncoso AP, Carpizo-Ituarte E, Pettay DT, Warner ME, Cupul-Magaña AL (2014) The effects of an abnormal decrease in temperature on the Eastern Pacific reef-building coral Pocillopora verrucosa. Mar Biol 161:131–139
Roth MS, Deheyn DD (2013) Effects of cold stress and heat stress on coral fluorescence in reef-building corals. Sci Rep 3:1421
Roth MS, Goericke R, Deheyn DD (2012) Cold induces acute stress but heat is ultimately more deleterious for the reef-building coral Acropora yongei. Sci Rep 2:240
Saxby T, Dennison WC, Hoegh-Guldberg O (2003) Photosynthetic responses of the coral Montipora digitata to cold temperature stress. Mar Ecol Prog Ser 248:85–97
Siebeck UE, Marshall NJ, Klüter A, Hoegh-Guldberg O (2006) Monitoring coral bleaching using a colour reference card. Coral Reefs 25:453–460
Sorensen JG, Dahlgaard J, Loeschcke V (2001) Genetic Variation in Thermal Tolerance among Natural Populations of Drosophila buzzatii: Down Regulation of Hsp70 Expression and Variation in Heat Stress Resistance Traits. Funct Ecol 15:289–296
Strahl J, Stolz I, Uthicke S, Vogel N, Noonan SHC, Fabricius KE (2015) Physiological and ecological performance differs in four coral taxa at a volcanic carbon dioxide seep. Comp Biochem Physiol -Part A Mol Integr Physiol 184:179–186
van Oppen MJH, Palstra FP, Piquet AMT, Miller DJ (2001) Patterns of coral-dinoflagellate associations in Acropora: significance of local availability and physiology of Symbiodinium strains and host-symbiont selectivity. Proc R Soc B Biol Sci 268:1759–1767
Vavrus S, Walsh JE, Chapman WL, Portis D (2006) The behavior of extreme cold air outbreaks under greenhouse warming. Int J Climatol 26:1133–1147
Visram S, Douglas AE (2007) Resilience and acclimation to bleaching stressors in the scleractinian coral Porites cylindrica. J Exp Mar Biol Ecol 349:35–44
Ward S (1995) Two patterns of energy allocation for growth, reproduction and lipid storage in the scleractinian coral Pocillopora damicornis. Coral Reefs 14:87–90
Weis V (2008) Cellular mechanisms of Cnidarian bleaching: stress cause the collapse of symbiosis. J Exp Biol 3059–3066
Whitlock MC, Schluter D (2009) The Analysis of Biological Data. Roberts and Comapny Publishers, Greenwod Village
Winters G, Holzman R, Blekhman A, Beer S, Loya Y (2009) Photographic assessment of coral chlorophyll contents: Implications for ecophysiological studies and coral monitoring. J Exp Mar Bio Ecol 380:25–35
Yamashiro H, Oku H, Onaga K (2005) Effect of bleaching on lipid content and composition of Okinawan corals. Fisheries Science 71:448–453
Yuyama I, Higuchi T (2014) Comparing the effects of symbiotic algae (Symbiodinium) clades C1 and D on early growth stages of Acropora tenuis. PLoS One 9:1–8
Acknowledgements
The authors wish to thank the staff at the National SeaSimulator Precinct at the Australian Institute of Marine Science for their generous help and expertise offered during the course of the experiment. This research was funded by internal funds from the Australian Institute of Marine Science. Student support and transport to AIMS was provided by AIMS@JCU.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interests.
Additional information
Topic Editor Mark Vermeij
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nielsen, J.J.V., Kenkel, C.D., Bourne, D.G. et al. Physiological effects of heat and cold exposure in the common reef coral Acropora millepora. Coral Reefs 39, 259–269 (2020). https://doi.org/10.1007/s00338-019-01881-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-019-01881-x