Abstract
As marine heatwaves increasingly threaten coral reefs worldwide, some extreme reef environments naturally expose corals to high-temperature fluctuations and can therefore provide important insights into the mechanisms underlying coral heat tolerance. Coral reefs in the Kimberley region in northwest Australia experience the world’s largest tropical tides and are therefore exposed to highly fluctuating temperatures in the intertidal. In contrast, the subtidal remains mostly submerged, resulting in moderate daily temperature fluctuations. A marine heatwave in 2016 triggered wide-spread bleaching in the Kimberley. Intertidal corals bleached less and recovered faster than adjacent subtidal corals; however, the mechanisms underlying this differential bleaching and recovery response remain poorly understood. Here we assessed both host- and symbiont-based indicators of bleaching resilience in the coral Acropora aspera. We tagged visibly healthy and bleached colonies from both environments in April 2016 and measured symbiont community composition, cell density, chlorophyll a, total biomass and host tissue energy reserves (lipids, protein and carbohydrates) during bleaching in April and in November 2016. Bleaching severity was higher in the subtidal than in intertidal, and while Cladocopium dominated all corals, symbiont community compositions differed significantly between environments and between bleached and healthy subtidal corals. Interestingly, bleaching resilience seemed decoupled from energy reserves, even though high levels of energy reserves and/or sufficient consumption during bleaching are widely thought to increase resistance to and recovery from bleaching. Although all bleached/recovered corals showed a general pattern of catabolizing protein reserves, distinct environment-specific trends were observed: subtidal corals that suffered extensive mortality also catabolized energy-poor carbohydrate reserves. In contrast, intertidal corals recovered rapidly after bleaching and maintained energy reserves. Total biomass remained unchanged between bleached and healthy corals in both environments. Overall, the findings of this study demonstrate that the consumption of energy reserves during bleaching is not always a reliable indicator of bleaching resilience.


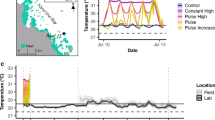
reproduced from Schoepf et al. 2020) and symbiont density (e, f) ± standard error (SE) of Acropora aspera. SE is missing for bleached/recovered subtidal corals in November 2016 as sample size was one (Table S3). Asterisks indicate significant differences between healthy and bleached/recovered corals within each environment. Significant environment (envo), health and time effects are indicated when present (Table S4). Note that the health status refers to the assessment in April 2016

Similar content being viewed by others
Data availability
Data will be submitted to www.pangea.de after publication.
References
Anthony KRN, Hoogenboom MO, Maynard JA, Grottoli AG, Middlebrook R (2009) Energetics approach to predicting mortality risk from environmental stress: a case study of coral bleaching. Funct Ecol 23:539–550
Baker AC (2001) Reef corals bleach to survive change. Nature 411:765–776
Bay LK, Doyle J, Logan M, Berkelmans R (2016) Recovery from bleaching is mediated by threshold densities of background thermo-tolerant symbiont types in a reef-building coral. Royal Society open science 3:160322
Berkelmans R, Van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proceedings of the Royal Society B: Biological Sciences 273:2305–2312
Camp EF, Schoepf V, Mumby PJ, Hardtke LA, Rodolfo-Metalpa R, Smith DJ, Suggett DJ (2018) The future of coral reefs subject to rapid climate change: lessons from natural extreme environments. Front Mar Sci 5:4
Cunning R, Ritson-Williams R, Gates RD (2016) Patterns of bleaching and recovery of Montipora capitata in Kāne ‘ohe Bay, Hawai ‘i, USA. Mar Ecol Prog Ser 551:131–139
Cunning R, Silverstein RN, Baker AC (2018) Symbiont shuffling linked to differential photochemical dynamics of Symbiodinium in three Caribbean reef corals. Coral Reefs 37:145–152
Dandan SS, Falter JL, Lowe RJ, McCulloch MT (2015) Resilience of coral calcification to extreme temperature variations in the Kimberley region, northwest Australia. Coral Reefs 34:1151–1163
Dubois M, Gilles KA, Hamilton JK, Rebers PAt, Smith F, (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Eren AM, Morrison HG, Lescault PJ, Reveillaud J, Vineis JH, Sogin ML (2015) Minimum entropy decomposition: unsupervised oligotyping for sensitive partitioning of high-throughput marker gene sequences. ISME J 9:968–979
Fisher R, O’Leary RA, Low-Choy S, Mengersen K, Knowlton N, Brainard RE, Caley MJ (2015) Species richness on coral reefs and the pursuit of convergent global estimates. Curr Biol 25:500–505
Fitt WK, McFarland FK, Warner ME, Chilcoat GC (2000) Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol Oceanogr 45:677–685
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Gilmour JP, Cook KL, Ryan NM, Puotinen ML, Green RH, Shedrawi G, Hobbs J-PA, Thomson DP, Babcock RC, Buckee J (2019) The state of Western Australia’s coral reefs. Coral Reefs 38:651–667
Gnaiger E, Bitterlich G (1984) Proximate biochemical composition and caloric content calculated from elemental CHN analysis: a stoichiometric concept. Oecologia 62:289–298
Goldberg WM (2018) Coral food, feeding, nutrition, and secretion: a review marine organisms as model systems in biology and medicine. Springer, pp 377–421
Goulet TL (2006) Most corals may not change their symbionts. Mar Ecol Prog Ser 321:1–7
Grottoli AG, Rodrigues LJ, Juarez C (2004) Lipids and stable carbon isotopes in two species of Hawaiian corals, Porites compressa and Montipora verrucosa, following a bleaching event. Mar Biol 145:621–631
Grottoli AG, Rodrigues LJ, Palardy JE (2006) Heterotrophic plasticity and resilience in bleached corals. Nature 440:1186–1189
Grottoli AG, Warner ME, Levas SJ, Aschaffenburg MD, Schoepf V, McGinley M, Baumann J, Matsui Y (2014) The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Glob Change Biol 20:3823–3833
Hillyer KE, Dias D, Lutz A, Roessner U, Davy SK (2018) 13 C metabolomics reveals widespread change in carbon fate during coral bleaching. Metabolomics 14:12
Houlbrèque F, Ferrier-Pagès C (2009) Heterotrophy in tropical scleractinian corals. Biol Rev 84:1–17
Howells EJ, Beltran VH, Larsen NW, Bay LK, Willis BL, Van Oppen MJH (2012) Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat Clim Chang 2:116–120
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83
Hughes TP, Kerry JT, Baird AH, Connolly SR, Chase TJ, Dietzel A, Hill T, Hoey AS, Hoogenboom MO, Jacobson M (2019) Global warming impairs stock–recruitment dynamics of corals. Nature 568:387–390
Hume BC, Smith EG, Ziegler M, Warrington HJ, Burt JA, LaJeunesse TC, Wiedenmann J, Voolstra CR (2019) SymPortal: a novel analytical framework and platform for coral algal symbiont next-generation sequencing ITS2 profiling. Mol Ecol Resour 19:1063–1080
Imbs AB, Yakovleva IM (2012) Dynamics of lipid and fatty acid composition of shallow-water corals under thermal stress: an experimental approach. Coral Reefs 31:41–53
Jeffrey SWt, Humphrey GF, (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Kenkel C, Meyer E, Matz M (2013) Gene expression under chronic heat stress in populations of the mustard hill coral (Porites astreoides) from different thermal environments. Mol Ecol 22:4322–4334
LaJeunesse TC, Smith RT, Finney J, Oxenford H (2009) Outbreak and persistence of opportunistic symbiotic dinoflagellates during the 2005 Caribbean mass coral ‘bleaching’ event. Proceedings of the Royal Society B: Biological Sciences 276:4139–4148
LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, Santos SR (2018) Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr Biol 28(2570–2580):e2576
Le Nohaïc M, Ross CL, Cornwall CE, Comeau S, Lowe R, McCulloch MT, Schoepf V (2017) Marine heatwave causes unprecedented regional mass bleaching of thermally resistant corals in northwestern Australia. Sci Rep 7:1–11
Levas SJ, Grottoli AG, Hughes A, Osburn CL, Matsui Y (2013) Physiological and biogeochemical traits of bleaching and recovery in the mounding species of coral Porites lobata: implications for resilience in mounding corals. PloS one 8(5):e63267
Morikawa MK, Palumbi SR (2019) Using naturally occurring climate resilient corals to construct bleaching-resistant nurseries. Proc Natl Acad Sci 116:10586–10591
Muscatine L, Cernichiari E (1969) Assimilation of photosynthetic products of zooxanthellae by a reef coral. Biol Bull 137:506–523
Muscatine L, Falkowski PG, Porter JW, Dubinsky Z (1984) Fate of photosynthetic fixed carbon in light-and shade-adapted colonies of the symbiotic coral Stylophora pistillata. Proc R Soc Lond B 222:181–202
Oliver TA, Palumbi SR (2011) Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30:429–440
Palumbi SR, Barshis DJ, Traylor-Knowles N, Bay RA (2014) Mechanisms of reef coral resistance to future climate change. Science 344:895–898
Parkinson JE, Banaszak AT, Altman NS, LaJeunesse TC, Baums IB (2015) Intraspecific diversity among partners drives functional variation in coral symbioses. Sci Rep 5:1–12
Pochon X, Pawlowski J, Zaninetti L, Rowan R (2001) High genetic diversity and relative specificity among Symbiodinium-like endosymbiotic dinoflagellates in soritid foraminiferans. Mar Biol 139:1069–1078
Richards ZT, Garcia RA, Wallace CC, Rosser NL, Muir PR (2015) A diverse assemblage of reef corals thriving in a dynamic intertidal reef setting (Bonaparte Archipelago, Kimberley, Australia). PLoS One. https://doi.org/10.1371/journal.pone.0117791
Richards ZT, Garcia R, Moore G, Fromont J, Kirkendale L, Bryce M, Bryce C, Hara A, Ritchie J, Gomez O (2019) A tropical Australian refuge for photosymbiotic benthic fauna. Coral Reefs 38:669–676
Rodrigues LJ, Grottoli AG (2007) Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnol Oceanogr 52:1874–1882
Rogers CS (1979) The effect of shading on coral reef structure and function. J Exp Mar Biol Ecol 41:269–288
Rosser NL, Veron JEN (2011) Australian corals thriving out of water in an extreme environment. Coral Reefs 30:21–21
Safaie A, Silbiger NJ, McClanahan TR, Pawlak G, Barshis DJ, Hench JL, Rogers JS, Williams GJ, Davis KA (2018) High frequency temperature variability reduces the risk of coral bleaching. Nat Commun 9:1–12
Schoepf V, Grottoli AG, Warner ME, Cai W-J, Melman TF, Hoadley KD, Pettay DT, Hu X, Li Q, Xu H (2013) Coral energy reserves and calcification in a high-CO2 world at two temperatures. PloS one. https://doi.org/10.1371/journal.pone.0075049
Schoepf V, Stat M, Falter JL, McCulloch MT (2015a) Limits to the thermal tolerance of corals adapted to a highly fluctuating, naturally extreme temperature environment. Sci Rep 5:1–14
Schoepf V, Grottoli AG, Levas SJ, Aschaffenburg MD, Baumann JH, Matsui Y, Warner ME (2015b) Annual coral bleaching and the long-term recovery capacity of coral. Proceedings of the Royal Society B: Biological Sciences 282:20151887
Schoepf V, Carrion SA, Pfeifer SM, Naugle M, Dugal L, Bruyn J, McCulloch MT (2019) Stress-resistant corals may not acclimatize to ocean warming but maintain heat tolerance under cooler temperatures. Nat Commun 10:1–10
Schoepf V, Jung MU, McCulloch MT, White N, Stat M, Thomas L (2020) Thermally variable, macrotidal reef habitats promote rapid recovery from mass coral bleaching. Front Mar Sci 7:245
Siebeck UE, Marshall NJ, Klüter A, Hoegh-Guldberg O (2006) Monitoring coral bleaching using a colour reference card. Coral Reefs 25:453–460
UE Siebeck, D Logan, NJ Marshall (2008) CoralWatch: a flexible coral bleaching monitoring tool for you and your group
Silverstein RN, Cunning R, Baker AC (2015) Change in algal symbiont communities after bleaching, not prior heat exposure, increases heat tolerance of reef corals. Glob Change Biol 21:236–249
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano M, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Analytical biochem 150(1):76–85
Spalding M, Burke L, Wood SA, Ashpole J, Hutchison J, Zu Ermgassen P (2017) Mapping the global value and distribution of coral reef tourism. Marine Policy 82:104–113
Stat M, Gates RD (2011) Clade D Symbiodinium in scleractinian corals: a “nugget” of hope, a selfish opportunist, an ominous sign, or all of the above? J Mar Bio. https://doi.org/10.1155/2011/730715
Stat M, Loh WKW, LaJeunesse TC, Hoegh-Guldberg O, Carter DA (2009) Stability of coral-endosymbiont associations during and after a thermal stress event in the southern Great Barrier Reef. Coral Reefs 28:709–713
Stimson JS (1987) Location, quantity and rate of change in quantity of lipids in tissue of Hawaiian hermatypic corals. Bull Mar Sci 41:889–904
Szmant A, Gassman NJ (1990) The effects of prolonged “bleaching” on the tissue biomass and reproduction of the reef coral Montastrea annularis. Coral Reefs 8:217–224
Thomas L, Kendrick GA, Kennington WJ, Richards ZT, Stat M (2014) Exploring Symbiodinium diversity and host specificity in Acropora corals from geographical extremes of Western Australia with 454 amplicon pyrosequencing. Mol Ecol 23:3113–3126
Thornhill DJ, Rotjan RD, Todd BD, Chilcoat GC, Iglesias-Prieto R, Kemp DW, LaJeunesse TC, Reynolds JM, Schmidt GW, Shannon T (2011) A connection between colony biomass and death in Caribbean reef-building corals. PLoS ONE 6:e29535
Wall CB, Ritson-Williams R, Popp BN, Gates RD (2019) Spatial variation in the biochemical and isotopic composition of corals during bleaching and recovery. Limnol Oceanogr 64:2011–2028
Ward S, Harrison P, Hoegh-Guldberg O (2002) Coral bleaching reduces reproduction of scleractinian corals and increases susceptibility to future stress. In: Proceedings of the ninth international coral reef symposium, Bali, 23–27 October 2000, vol 2. pp 1123–1128
Warner ME, Fitt WK, Schmidt GW (1996) The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: a novel approach. Plant, Cell Environ 19:291–299
Acknowledgements
We thank C. Cornwall, S. Comeau, A. Kuret, A.-M. Nisumaa-Comeau, D. Thompson, M. Le Nohaïc , X. Chen, T. DeCarlo, J. Brown, G. Firman and the staff at Cygnet Bay Pearl Farm for field and laboratory assistance, A. Meenakshisundaram for assistance with genetic data analyses and Ben Hume for assistance with running samples through SymPortal. We especially thank the Bardi Jawi people who enabled this research through their advice and consent to access their traditional lands.
Funding
This study was funded by the PADI Foundation (Research Grant #21737), the University of Western Australia (Research Collaboration Award) and the Australian Research Council Centre of Excellence for Coral Reef Studies (CE140100020).
Author information
Authors and Affiliations
Contributions
VS designed the study and led the field work. MJ conducted the physiological analyses, while LT, MJ, AK and MS conducted the genetic analyses. MJ executed the data analyses and statistical analyses with input from VS, LT and MS. MJ led the writing of the paper, with all co-authors contributing to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that no conflict of interest exists.
Ethical approval
Corals were collected using exemption #2549 from the Western Australia Department of Fisheries.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic editor Simon Davy
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jung, E.M.U., Stat, M., Thomas, L. et al. Coral host physiology and symbiont dynamics associated with differential recovery from mass bleaching in an extreme, macro-tidal reef environment in northwest Australia. Coral Reefs 40, 893–905 (2021). https://doi.org/10.1007/s00338-021-02094-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-021-02094-x