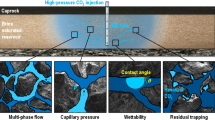
Abstract
In the present study, various cap rocks have been experimentally reacted in water with supercritical CO2 in geological storage conditions (P = 8 × 106 Pa and T = 80 °C) for 25 days. To characterize the potential CO2–water–rock interactions, an experimental setup has been built to provide additional information concerning the effects of structure, thermal and surface characteristics changes due to CO2 injection with cap rocks. In addition, CO2 adsorption capacities of different materials (i.e., clay evaporate and sandstone) are measured. These samples were characterized by XRD technique. The BET specific surface area was determined by nitrogen isotherms. In addition, thermal characteristics of untreated adsorbents were analyzed via TGA method and topography surfaces are identified by Scanning Electron Microscope (SEM). Taking into account pressure and temperature, the physical as well as chemical mechanisms of CO2 retention were determined. Isotherm change profiles of samples for relative pressure range indicate clearly that CO2 was adsorbed in different quantities. In accordance with the X-ray diffraction, a crystalline phase was formed due to the carbonic acid attack and precipitation of some carbonate.










Similar content being viewed by others
References
P. Soares, Warming Power of CO2 and H2O: Correlations with Temperature Changes, Int. J. Geosci. 1, 102–112 (2010)
P. Jean-Baptiste, R. Ducroux, Potentiel des méthodes de séparation et stockage du CO2 dans la lutte contre l’effet de serre, C. R. Geosci. 335, 611–625 (2003)
R. Qi, T, C. L, Martin, J. Blunt. Design of carbon dioxide storage in aquifers, Int. J. Greenh. Gas Control 3, 195–205(2009)
N. Kampman, M. Bickle, M. Wigley, B. Dubacq, Fluid flow and CO2–fluid–mineral interactions during CO2-storage in sedimentary basins. Chem. Geol. 369, 22–50 (2014)
S. Bachu, Sequestration of CO2 in geological media in response to climate change:road map for site selection using the transform of the geological space into the CO2 phase space, Energy Convers. Manage. 43, 87–102 (2002)
I. Gaus, Role and impact of CO2–rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control 4, 73–89 (2010)
F. Guyot, D. Daval, S. Dupraz, I. Martinez, B. Ménez, O. Sissmann, CO2 geological storage: The environmental mineralogy perspective, C. R. Geosci. 343, 246–259 (2011)
D.F. Zhao, X.W. Liao, D.D. Yin, An experimental study for the effect of CO2–brine–rock interaction on reservoir physical properties. J. Energy Inst. 88, 27–35 (2015)
M. Wdowin, R. Tarkowski, W. Franus, Determination of changes in the reservoir and cap rocks of the Chabowo Anticline caused by CO2–brine–rock interactions. Int. J. Coal Geol. 130, 79–88 (2014)
A. Olabode, M. Radonjic, Experimental investigations of caprock Integrity in CO2 sequestration, Energy Procedia 37, 5014–5025 (2013)
E. Galán, P. Aparicio, Experimental study on the role of clays as sealing materials in the geological storage of carbon dioxide. Appl. Clay Sci. 87, 22–27 (2014)
T. Wang, H. Wang, F. Zhang, T. Xu, Simulation of CO2–water–rock interactions on geologic CO2 sequestration under geological conditions of China. Mar. Pollut. Bull. 76, 307–314 (2013)
P. Jeon, J. Choi, T. Yun, C. Lee, Sorption equilibrium and kinetics of CO2 on clay minerals from subcritical to supercritical conditions: CO2 sequestration at nanoscale interfaces. Chem. Eng. J. 255, 705–715 (2014)
Y. Soong, B H. Howard, S W. Hedges, I. Haljasmaa, R P. Warzinski, G. Irdi, T R. McLendon, CO2 sequestration in saline formation. Aerosol. Air Qual. Res. 14, 522–532 (2014)
K. Wang, T. Xu, F. Wang, H. Tian, Experimental study of CO2–brine–rock interaction during CO2 sequestration in deep coal seams. Int. J. Coal Geol. 154–155, 265–274 (2016)
S. Kamel, Application of selected geothermometers to Continental Intercalaire thermal water in southern Tunisia, Geothermics 41, 63–73 (2012)
S. Storck, H. Bretinger, W. Maier, Characterization of micro- and mesoporous solids by physisorption methods and pore-size analysis, Appl. Catal. A General 174,137–146 (1998)
S. Lee, S. Park, Determination of the optimal pore size for improved CO2 adsorption in activated carbon fibers. J. Colloid Interface Sci. 389, 230–235 (2013)
A. Heidari, H. Younesi, A. Rashidi, A. Ghoreyshi. Adsorptive removal of CO2 on highly microporous activated carbons prepared from Eucalyptus camaldulensis wood: Effect of chemical activation, J. Taiwan Instit. Chem. Eng. 45, 579–588 (2014)
R. Xun, M. Jia, F. Li, H. Wang, B. Zhang, J. Qiao, Appl. Phys. A 106, 747–755 (2012)
F. Kooli, Y. Liu, R. Al-Faze, A. Suhaimi, Effect of acid activation of Saudi local clay mineral on removal properties of basic blue 41 from an aqueous solution, Appl. Clay Sci. 116–117, 23–30 (2015)
H. Lu, X. Yang, G. Gao, K. Wang, Q. Shi, J. Wang, C. Han, J. Liu, M. Tong, X. Liang, C. Li, Mesoporous zirconia-modified clays supported nickel catalysts for CO and CO2 methanation, Int. J. Hydrogen Energy 39, 18894–18907 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jedli, H., Jbara, A., Hedfi, H. et al. A laboratory study of supercritical CO2 adsorption on cap rocks in the geological storage conditions. Appl. Phys. A 123, 254 (2017). https://doi.org/10.1007/s00339-017-0862-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-017-0862-0