Abstract
Purpose
Benign prostatic hyperplasia (BPH) is associated with lower urinary tract symptoms (LUTS), representing one of the most common urological conditions. However, insights into the actual healthcare of this patient cohort in Germany are scarce. We aimed to retrospectively analyse management patterns of patients with LUTS in Germany using health insurance claims databases.
Methods
A retrospective, longitudinal cohort analysis was conducted obtaining claims data from the German InGef health insurance database containing approximately five million member-records from over 60 nationwide statutory health insurances. First, a cross-sectional prevalence analysis was performed on all individuals with a diagnosis on LUTS (ICD-10 GM N40) in 2018. Second, a longitudinal analysis of individuals with either a newly started BPH medication or initial BPH surgery who were indexed in 2014 and followed-up for 4 years.
Results
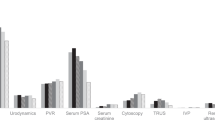
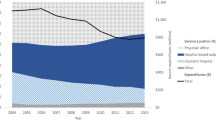
In 2018, 132,386 (6.7%) prevalent BPH patients were identified from 1,979,916 continuously insured males. A potential overcoding bias could not be assessed which may influence the outpatient sector estimation. 10,361 (0.7%) patients were identified with incident BPH medication and 1768 (0.1%) patients with incident BPH surgery out of 1,575,604 males (2013–2018). Alpha-blockers were the drug of choice (95.6%) in the first year. Half of patients received specific BPH medications four years after index, while almost 98% of initial BPH surgeries were performed within the inpatient setting. TURP was the most frequent surgical intervention (76%).
Conclusions
A widespread diffusion of alternative individualized minimally invasive approaches in the outpatient sector might address pharmacotherapy discontinuation and patient-access barriers to other treatments.
Similar content being viewed by others
References
Vuichoud C, Loughlin KR (2015) Benign prostatic hyperplasia: epidemiology, economics and evaluation. Can J Urol 22(Suppl 1):1–6
Luca C et al (2015) Patient’s adherence on pharmacological therapy for benign prostatic hyperplasia (BPH)-associated lower urinary tract symptoms (LUTS) is different: is combination therapy better than monotherapy? BMC Urol 15(1):96. https://doi.org/10.1186/s12894-015-0090-x
Gilfrich C, Leicht H, Fahlenbrach C, Jeschke E, Popken G, Stolzenburg JU, Weißbach L, Zastrow C, Günster C (2016) Morbidity and mortality after surgery for lower urinary tract symptoms: a study of 95 577 cases from a nationwide German health insurance database. Prostate Cancer Prostat Dis 19(4):406–411. https://doi.org/10.1038/pcan.2016.33 (Epub 2016 Aug 9 PMID: 27502738)
Chung ASJ, Woo HH (2018) Update on minimally invasive surgery and benign prostatic hyperplasia. Asian J Urol 5(1):22–27. https://doi.org/10.1016/j.ajur.2017.06.001
Lee SWH, Chan EMC, Lai YK (2017) The global burden of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a systematic review and meta-analysis. Sci Rep 7(1):1–10. https://doi.org/10.1038/s41598-017-06628-8
Russo GI, Urzì D, Cimino S (2018) Epidemiology of LUTS and BPH. Lower urinary tract symptoms and benign prostatic hyperplasia. Academic Press, Cambridge, pp 1–14
Andersohn F, Walker J (2016) Characteristics and external validity of the German health risk institute (HRI) database. Pharmacoepidemiol Drug Saf 25(1):106–109. https://doi.org/10.1002/pds.3895
Berges R (2008) Epidemiologie des benignen prostatasyndroms. Urologe 47(2):141–148
Carballido J et al (2011) Can benign prostatic hyperplasia be identified in the primary care setting using only simple tests? Results of the diagnosis improvement in primary care trial. Int J Clin Pract 65(9):989–996. https://doi.org/10.1111/j.1742-1241.2011.02735.x
Lee C-L, Kuo H-C (2017) Current consensus and controversy on the diagnosis of male lower urinary tract symptoms/benign prostatic hyperplasia. Tzu-Chi Med J 29(1):6. https://doi.org/10.4103/tcmj.tcmj_3_17
Cindolo L, Pirozzi L, Fanizza C et al (2015) Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: population-based cohort study. Eur Urol 68(3):418–425. https://doi.org/10.1016/j.eururo.2014.11.006
Malaeb BS, Yu X, McBean AM, Elliott SP (2012) National trends in surgical therapy for benign prostatic hyperplasia in the United States (2000–2008). Urology 79(5):1111–1116. https://doi.org/10.1016/j.urology.2011.11.084
Jeon BJ, Chung H, Bae JH, Jung H, Lee JG, Choi H (2019) Analysis of present status for surgery of benign prostatic hyperplasia in korea using nationwide healthcare system data. Int Neurourol J 23(1):22–29. https://doi.org/10.5213/inj.1836198.099
Campbell J et al (2019) The utilization of benign prostatic hyperplasia and bladder-related medications after a transurethral prostatectomy. Urology 130:126–131. https://doi.org/10.1016/j.urology.2019.05.003
Han HH, Ko WJ, Yoo TK, Oh TH, Kim DY, Kwon DD, Byun SS, Kim SI, Jung TY (2014) Factors associated with continuing medical therapy after transurethral resection of prostate. Urology 84(3):675–680. https://doi.org/10.1016/j.urology.2014.04.027 (Epub 2014 Jul 22 PMID: 25059592)
De Nunzio C et al (2018) Patient centred care for the medical treatment of lower urinary tract symptoms in patients with benign prostatic obstruction: a key point to improve patients’ care–a systematic review. BMC Urol 18(1):62. https://doi.org/10.1186/s12894-018-0376-x
Rassweiler J et al (2006) Complications of transurethral resection of the prostate (TURP)—incidence, management, and prevention. Eur Urol 50(5):969–980. https://doi.org/10.1016/j.eururo.2005.12.042
Palmisano F et al (2018) Incidence and predictors of readmission within 30 days of transurethral resection of the prostate: a single center European experience. Sci Rep 8(1):1–7. https://doi.org/10.1038/s41598-018-25069-5
Standl T, Lussi C (eds) (2016) Ambulantes Operieren in Klinik, Praxis und MVZ: Rahmenbedingungen-Organisation-Patientenversorgung. Springer-Verlag, Berlin
Miner MM (2009) Primary care physician versus urologist: how does their medical management of LUTS associated with BPH differ? Curr Urol Rep 10(4):254–260. https://doi.org/10.1007/s11934-009-0042-7
Rosenberg MT et al (2013) The evaluation and treatment of prostate-related LUTS in the primary care setting: the next STEP. Curr Urol Rep 14(6):595–605. https://doi.org/10.1007/s11934-013-0371-4
Author information
Authors and Affiliations
Contributions
AM: protocol/project development; data analysis, manuscript writing/editing; JF: data analysis, manuscript writing/editing; BL: protocol/project development; data analysis, data collection or management, manuscript writing/editing; IK: data analysis, manuscript writing/editing; VM: data analysis, manuscript writing/editing; DM: data collection or management, data analysis, manuscript writing/editing; MM: protocol/project development; data collection or management, data analysis; CG: protocol/project development; data analysis; RS: protocol/project development; data analysis, manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of interest
Prof. Dr. med. Christian Gratzke is advisor for Astellas Pharma GmbH, DE, Ipsen Pharma GmbH, DE, Steba Biotech S.A., LUX, Bayer Pharma, DE, Olympus Winter & Ibe GmbH, DE, Medi-Tate Ltd., IL, MSD, DE, Astra-Zeneca, UK and Roche, CH. He receives speaker fees from Amgen, USA, Astellas Pharma GmbH, DE, Ipsen Pharma GmbH, DE, Janssen-Cilag GmbH, BEL, Bayer Pharma, DE, Takeda Pharmaceuticals, JPN and medac GmbH, DE. Prof. Dr. med. Arkadiusz Miernik has received research funding from the Federal Ministry of Education and Research (BMBF), DE, coverage of travel expenses from the German Association of Urology (DGU), DE, European Association of Urology (EAU), NL; he is advisor for KLS Martin GmbH, DE, Dornier MedTech Europe GmbH, RichardWolf GmbH, DE, KarlStorz SE & Co. KG, DE, Lisa laser OHG, DE, Boston Scientific, USA, Dornier MedTech Europe GmbH, DE, Medi-Tate Ltd., IL and reviewer for Ludwig Boltzmann Gesellschaft, A; he has royalties from Walter de Gruyter, DE, Springer Science + Business Media, DE. Dr. med. Jonas Fritzsche has no conflict of interest to declare. At the time to conduction of this study Berit Libutzki, Melanie May and Damon Mohebbi were employees of HGC Healthcare Consultants GmbH, which received funding from Medi-Tate Ltd. to conduct this study. Vanessa Malka and Ido Kilemnik are employed at Medi-Tate Ltd. Dr. med. Rodrigo Suarez has no conflicts of interests to declare.
Human participants and/or animals
This analysis is based on retrospective claims data of individuals insured within the German statutory health insurance. All individual patient data were de-identified to comply with German federal data protection regulations. Due to data protection regulations, patient numbers below five were not displayed.
Informed consent
The analysis did not involve decisions regarding interventions or the omission of interventions, therefore, institutional review board/ethical approval and patient informed consent was not needed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miernik, A., Fritzsche, J., Libutzki, B. et al. Real-world data and treatment patterns of patients with lower urinary tract symptoms due to benign prostatic hyperplasia in Germany: an observational study using health insurance claims data. World J Urol 39, 4381–4388 (2021). https://doi.org/10.1007/s00345-021-03787-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-021-03787-2