Abstract
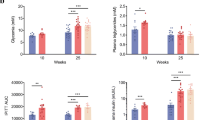
Diabetic cardiomyopathy (DCM) is a disease of heart muscle that remains one of the leading causes of death in diabetic individuals. Shifts in substrate preference resulting in aberrant serum lipid content and enlarged left ventricular wall thickness are well-established characteristics associated with the development of DCM. As underlying mechanisms driving the onset of the DCM remain relatively unclear, this study sought to characterize age-dependent development of left ventricular (LV) wall thickness in diabetic (db/db) mice. Such data were compared with low-density lipoprotein (LDL) and triglyceride serum levels to assess whether any correlation exists between the parameters here investigated. For methods, db/db mice together with nondiabetic controls (n = six per group) were monitored from the age of 6–16 weeks. Mice were terminated each week to measure body weights, heart weights, liver weights, tibia length, and fasting plasma glucose levels. Heart tissues were stained with haematoxylin and eosin to measure LV wall and interventricular septum thickness together with an assessment of myocardial remodeling. Serum was collected weekly and used to measure LDL and triglyceride levels. Results showed that db/db mice presented significantly increased body weights, liver/body weight, and fasting plasma glucose levels from the age of 6–16 weeks. They further displayed a marked enlargement of LV wall and interventricular septum thickness from the age of 11 weeks, while increased heart weight/tibia length was recorded only from week 16. From week 11, the LV wall and interventricular septum thickness results corresponded with cardiac remodeling and raised LDL and triglyceride serum levels. In summary, age-dependent development of LV wall thickness in db/db mice is partially associated with increased LDL and triglyceride levels, elucidating a potential pathophysiological mechanism.





Similar content being viewed by others
References
Dandamudi S, Slusser J, Mahoney DW, Redfield MM, Rodeheffer RJ, Chen HH (2014) The prevalence of diabetic cardiomyopathy: a population-based study in Olmsted County, Minnesota. J Card Fail 20:304–309
Miki T, Yuda S, Kouzu H, Miura T (2013) Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev 18:149–166
Fujiwara T, Yoshida M, Nakamura T, Sakakura K, Wada H, Arao K, Katayama T, Funayama H, Sugawara Y, Mitsuhashi T, Kakei M, Momomura S, Ako J (2015) Dipeptidyl peptidase-4 inhibitors are associated with improved left ventricular diastolic function after acute myocardial infarction in diabetic patients. Heart Vessels 30:696–701
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30:595–602
Poornima IG, Parikh P, Shannon RP (2006) Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res 98:596–605
Boudina S, Abel ED (2010) Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord 11:31–39
Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV (2000) Impact of diabetes on cardiac structure and function: the strong heart study. Circulation 101:2271–2276
De Simone G, Palmieri V, Bella JN, Celentano A, Hong Y, Oberman A (2002) Association of left ventricular hypertrophy with metabolic risk factors: the HyperGEN study. J Hypertens 20:323–331
Cassidy S, Hallsworth K, Thoma C, MacGowan GA, Hollingsworth KG, Day CP, Taylor R, Jakovljevic DG, Trenell MI (2015) Cardiac structure and function are altered in type 2 diabetes and non-alcoholic fatty liver disease and associate with glycemic control. Cardiovasc Diabetol 14:23
Adeghate E, Singh J (2014) Structural changes in the myocardium during diabetes-induced cardiomyopathy. Heart Fail Rev 19:15–23
Stanley WC, Recchia FA, Lopaschuk GD (2005) Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85:1093–1129
Lionetti V, Stanley WC, Recchia FA (2011) Modulating fatty acid oxidation in heart failure. Cardiovasc Res 90:202–209
Wende AR, Symons JD, Abel ED (2012) Mechanisms of lipotoxicity in the cardiovascular system. Curr Hypertens Rep 14:517–531
Marfella R, Di Filippo C, Portoghese M, Barbieri M, Ferraraccio F, Siniscalchi M, Cacciapuoti F, Rossi F, D’Amico M, Paolisso G (2009) Myocardial lipid accumulation in patients with pressure-overloaded heart and metabolic syndrome. J Lipid Res 50:2314–2323
DeFronzo RA (2010) Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 53:1270–1287
Belke DD, Larsen TS, Gibbs EM, Severson DL (2000) Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab 279:E1104–E1113
Hall ME, Harmancey R, Stec DE (2015) Lean heart: role of leptin in cardiac hypertrophy and metabolism. World J Cardiol 7:511–524
Abel ED, Litwin SE, Sweeney G (2008) Cardiac remodeling in obesity. Physiol Rev 88:389–419
Kawano A, Nakamura H, Hata S, Minakawa M, Miura Y, Yagasaki K (2009) Hypoglycemic effect of aspalathin, a rooibos tea component from Aspalathus linearis, in type 2 diabetic model db/db mice. Phytomedicine 16:437–443
Zheng A, Li H, Xu J, Cao K, Li H, Pu W, Yang Z, Peng Y, Long J, Liu J, Feng Z (2015) Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: role of AMP-activated protein kinase activation. Br J Nutr 113:1667–1676
Page BJ, du Toit DF, Muller CJ, Mattysen J, Lyners R, Arends E (2004) Autogenous transplantation of a duct ligated pancreas: a functional and histological study. JOP 5:71–80
Kobayashi K, Forte TM, Taniguchi S, Ishida BY, Oka K, Chan L (2000) The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism 49:22–31
Hummel KP, Coleman DL, Lane PW (1972) The influence of genetic background on expression of mutations at the diabetes locus in the mouse. I. C57BL/KsJ and C57BL/6J strains. Biochem Genet 7:1–13
Paschos P, Paletas K (2009) Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia 13:9–19
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752
Ali MK, Narayan KMV, Tandon N (2010) Diabetes and coronary heart disease: current perspectives. Indian J Med Res 132:584–597
Yin FC, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG (1982) Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am J Physiol 243:H941–H947
Li RJ, Yang J, Yang Y, Ma N, Jiang B, Sun QW, Li YJ (2014) Speckle tracking echocardiography in the diagnosis of early left ventricular systolic dysfunction in type II diabetic mice. BMC Cardiovasc Disord 14:141
Barouch LA, Berkowitz DE, Harrison RW, O’Donnell CP, Hare JM (2003) Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation 108:754–759
Dludla PV, Muller CJF, Joubert E, Louw J, Essop MF, Gabuza KB, Ghoor S, Huisamen B, Johnson R (2017) Aspalathin protects the heart against hyperglycemia-induced oxidative damage by up-regulating Nrf2 expression. Molecules. doi:10.3390/molecules22010129
Sai E, Shimada K, Yokoyama T, Hiki M, Sato S, Hamasaki N, Maruyama M, Morimoto R, Miyazaki T, Fujimoto S, Tamura Y, Aoki S, Watada H, Kawamori R, Daida H (2017) Myocardial triglyceride content in patients with left ventricular hypertrophy: comparison between hypertensive heart disease and hypertrophic cardiomyopathy. Heart Vessels 32:166–174
Shin YH, Min JJ, Lee JH, Kim EH, Kim GE, Kim MH, Lee JJ, Ahn HJ (2016) The effect of fluvastatin on cardiac fibrosis and angiotensin-converting enzyme-2 expression in glucose-controlled diabetic rat hearts. Heart Vessels. doi:10.1007/s00380-016-0936-5
Johnson R, Dludla P, Joubert E, February F, Mazibuko S, Ghoor S, Muller C, Louw J (2016) Aspalathin, a dihydrochalcone C-glucoside, protects H9c2 cardiomyocytes against high glucose-induced shifts in substrate preference and apoptosis. Mol Nutr Food Res 60:922–934
Dludla PV, Muller CJF, Joubert E, Louw J, Gabuza KB, Huisamen B, Essop MF, Johnson R (2016) Phenylpyruvic acid-2-O-β-d-glucoside attenuates high glucose-induced apoptosis in H9c2 cardiomyocytes. Planta Med 82:1468–1474
Acknowledgements
This research was funded in part by the National Research Foundation (NRF) Thuthuka Programme Grant 87836 and the South Africa Medical Research Council’s Biomedical Research and Innovation Platform. The grantholders acknowledge that opinions, findings, and conclusions or recommendations expressed in any publication generated by the NRF supported research are those of the authors, and that the NRF accepts no liability whatsoever in this regard. Funding from Stellenbosch University and Ernst Ethel Erikson trust is also acknowledged. We would like to thank Charna Chapman, Desmond Linden, and Joritha van Heerden for technical support with animal work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Rights and permissions
About this article
Cite this article
Dludla, P.V., Essop, M.F., Gabuza, K.B. et al. Age-dependent development of left ventricular wall thickness in type 2 diabetic (db/db) mice is associated with elevated low-density lipoprotein and triglyceride serum levels. Heart Vessels 32, 1025–1031 (2017). https://doi.org/10.1007/s00380-017-0978-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-017-0978-3