Abstract
Aim
This study investigated whether dietary protein intake less (50%) or greater (250%) than requirements throughout gestation differently affects offspring body composition and cellular properties of skeletal muscle and subcutaneous adipose tissue (SCAT).
Methods
Primiparous gilts were fed iso-energetic diets containing adequate (22 AP), high (21 HP), or low (19 LP) protein contents. Newborn (n = 166) and weanling piglets cross-fostered to sows fed a standard diet (day 28; n = 83) were examined by morphological, biochemical, histological, and molecular analyses of the body, SCAT, and semitendinosus, longissimus, biceps femoris muscles.
Results
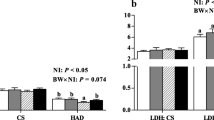
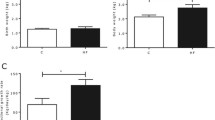
Lowered birth weight (BW) in response to the HP and LP diets (p < 0.01) resulted from decreases in all body constituents in LP, and mainly from reduced body fat in HP piglets (p < 0.05). In the light BW class within litters, HP piglets exhibited a greater percentage of muscle tissue (p < 0.05) than LP piglets. Less SCAT mass in HP and LP piglets resulted from reduced (p < 0.05) number, but not the size of adipocytes. The LP diet adversely affected myogenesis and muscular differentiation derived from less (p < 0.01) primary and secondary myofibers, lower creatine kinase activity (p < 0.05), less IGF2 mRNA (p < 0.10), and greater expression of the embryonic myosin heavy chain isoform (p < 0.01). Catch-up growth of LP but not HP pigs until day 28 increased body fat (p = 0.01). Despite compensated muscle growth in LP piglets, the deficit in myofiber number remained.
Conclusion
Poor intrauterine environment by limited and excess protein supply retards fetal growth, but only limited protein supply impairs myogenesis, persistently restricts muscle growth potential, and favors obesity at infancy.

Similar content being viewed by others
References
Gluckman PD, Hanson MA, Mitchell MD (2010) Developmental origins of health and disease: reducing the burden of chronic disease in the next generation. Genome Med 2:14
Hales CN, Barker DJ (1992) Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35:595–601
Gluckman PD, Hanson MA, Spencer HG (2005) Predictive adaptive responses and human evolution. Trends Ecol Evol 20:527–533
Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359:61–73
Wu G, Bazer FW, Wallace JM, Spencer TE (2006) Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci 84:2316–2337
Litten JC, Drury PC, Corson AM, Lean IJ, Clarke L (2003) The influence of piglet birth weight on physical and behavioural development in early life. Biol Neonate 84:311–318
Gondret F, Lefaucheur L, Juin H, Louveau I, Lebret B (2006) Low birth weight is associated with enlarged muscle fiber area and impaired meat tenderness of the longissimus muscle in pigs. J Anim Sci 84:93–103
Rehfeldt C, Kuhn G (2006) Consequences of birth weight for postnatal growth performance and carcass quality in pigs as related to myogenesis. J Anim Sci 84(Suppl):E113–E123
Rehfeldt C, Tuchscherer A, Hartung M, Kuhn G (2008) A second look at the influence of birth weight on carcass and meat quality in pigs. Meat Sci 78:170–175
Dwyer CM, Stickland NC (1991) Sources of variation in myofiber number within and between litters of pigs. Anim Prod 52:527–533
Langley-Evans SC (2009) Nutritional programming of disease: unravelling the mechanism. J Anat 215:36–51
Mahan DC (1977) Effect of feeding various gestation and lactation dietary protein sequences on long-term reproductive performance in swine. J Anim Sci 45:1061–1072
Pond WG, Maurer RR, Mersmann HJ, Cummins S (1992) Response of fetal and newborn piglets to maternal protein restriction during early or late pregnancy. Growth Dev Aging 56:115–127
Schoknecht PA, Newton GR, Weise DE, Pond WG (1994) Protein restriction in early pregnancy alters fetal and placental growth and allantoic fluid proteins in swine. Theriogenology 42:217–226
Pond WG, Yen JT, Mersmann HJ (1987) Effect of severe dietary protein, nonprotein calories or feed restriction during gestation on postnatal growth of progeny in swine. Growth 51:355–371
Pond WG, Yen JT, Mersmann HJ, Maurer RR (1990) Reduced mature size in progeny of swine severely restricted in protein intake during pregnancy. Growth Dev Aging 54:77–84
Pond WG, Maurer RR, Klindt J (1991) Fetal organ response to maternal protein deprivation during pregnancy in swine. J Nutr 121:504–509
Schoknecht PA, Pond WG, Mersmann HJ, Maurer RR (1993) Protein restriction during pregnancy affects postnatal growth in swine progeny. J Nutr 123:1818–1825
Sayer AA, Stewart C, Patel H, Cooper C (2010) The developmental origins of sarcopenia: from epidemiological evidence to underlying mechanisms. JDOHaD 1:150–157
Daenzer M, Ortmann S, Klaus S, Metges CC (2002) Prenatal high protein exposure decreases energy expenditure and increases adiposity in young rats. J Nutr 132:142–144
Thone-Reineke C, Kalk P, Dorn M, Klaus S, Simon K, Pfab T, Godes M, Persson P, Unger T, Hocher B (2006) High-protein nutrition during pregnancy and lactation programs blood pressure, food efficiency, and body weight of the offspring in a sex-dependent manner. Am J Physiol Regul Integr Comp Physiol 291:R1025–R1030
Kucia M, Langhammer M, Görs S, Albrecht E, Hammon HM, Nürnberg G, Metges CC (2011) High protein diet during gestation and lactation affects mammary gland mRNA abundance, milk composition and pre-weaning litter growth in mice. Animal 5:268–277
Campbell-Brown M, Johnstone FD, Kerr-Grieve JF (1986) The effect on birthweight of a high-protein, low carbohydrate diet during pregnancy. Proc Nutr Soc 45:90A (Abstr.)
Andreasyan K, Ponsonby AL, Dwyer T, Morley R, Riley M, Dear K, Cochrane J (2007) Higher maternal dietary protein intake in late pregnancy is associated with a lower infant ponderal index at birth. Eur J Clin Nutr 61:498–508
Moran LJ, Noakes M, Clifton PM, Tomlinson L, Galletly C, Norman RJ (2003) Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab 88:812–819
Rehfeldt C, Lang IS, Görs S, Hennig U, Kalbe C, Stabenow B, Brüssow KP, Pfuhl R, Bellmann O, Nürnberg G, Otten W, Metges CC (2011) Low and excess dietary protein levels during gestation affect growth and compositional traits in gilts and impair offspring fetal growth. J Anim Sci 89:329–341
GfE (Gesellschaft für Ernährungsphysiologie) (2006) Empfehlungen zur Energie- und Nährstoffversorgung von Schweinen (Recommendations of energy and nutrient intake in pigs). DLG-Verlags-GmbH, Frankfurt am Main
AOAC (1990) Official methods of analysis, 15th edn. Assoc. Off. Anal. Chem, Washington, DC
Kuhn G, Ender K, Nürnberg K (1994) Effects of recombinant porcine somatotropin on the chemical composition of the whole edible body at pigs. Arch Anim Breed 37:621–623
Lösel D, Nürnberg G, Rehfeldt C (2011) Regional differences in micro-structural and biochemical characteristics of growth and metabolism in semitendinosus muscle of 28-day old piglets. Meat Sci 87:19–25
Romeis B (1989) Mikroskopische Technik. Urban & Schwarzenberg, Munich
Guth L, Samaha FJ (1970) Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol 28:365–367
Rehfeldt C, Kuhn G, Vanselow J, Fürbass R, Fiedler I, Nürnberg G, Clelland AK, Stickland NC, Ender K (2001) Maternal treatment with somatotropin during early gestation affects basic events of myogenesis in pigs. Cell Tissue Res 306:429–440
Spannhof L (1967) Einführung in die Praxis der Histochemie. VEB Gustav-Fischer-Verlag, Jena
Novikoff AB, Shin WY, Drucker J (1961) Mitochondrial localization of oxidative enzymes: staining results with two tetrazolium salts. J Biophys Biochem Cytol 9:47–61
Rehfeldt C, Walther K (1997) A combined assay for DNA, protein, and incorporated [3H] label in cultured muscle cells. Anal Biochem 251:294–297
Oksbjerg N, Petersen JS, Sorensen IL, Henckel P, Vestergaard M, Ertbjerg P, Moller AJ, Bejerholm C, Stoier S (2000) Long-term changes in performance and meat quality of Danish Landrace pigs: a study on a current compared with an unimproved genotype. Anim Sci 71:81–92
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–356
Talmadge RJ, Roy RR (1993) Electrophoretic separation of rat skeletal muscle myosin heavy chain isoforms. J Appl Physiol 75:2337–2340
Lefaucheur L, Ecolan P, Lossec G, Gabillard JC, Butler-Browne GS, Herpin P (2001) Influence of early postnatal cold exposure on myofiber maturation in pig skeletal muscle. J Muscle Res Cell Motil 22:439–452
Kalbe C, Puppe B (2010) Long-term cognitive enrichment affects opioid receptor expression in the amygdala of domestic pigs. Genes Brain Behav 9:75–83
Erkens T, van Poucke M, Vandesompele J, Goossens K, van Zeveren A, Peelman LJ (2006) Development of a new set of reference genes for normalization of real-time RT-PCR data of porcine backfat and longissimus dorsi muscle, and evaluation with PPARGCIA. BMC Biotechnol 6:41
Langley-Evans SC, Gardner DS, Jackson AA (1996) Association of disproportionate growth of fetal rats in late gestation with raised systolic blood pressure in later life. J Reprod Fertil 106:307–312
Morise A, Seve B, Mace K, Magliola C, Le Huerou-luron I, Louveau I (2009) Impact of intrauterine growth retardation and early protein intake on growth, adipose tissue, and the insulin-like growth factor system in piglets. Pediatr Res 65:45–50
Halton TL, Hu FB (2004) The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr 23:373–385
Kuhla B, Kucia M, Gors S, Albrecht D, Langhammer M, Kuhla S, Metges CC (2010) Effect of a high-protein diet on food intake and liver metabolism during pregnancy, lactation and after weaning in mice. Proteomics 10:2573–2588
Görs S, Lang IS, Stabenow B, Hennig U, Brüssow K, Otten W, Rehfeldt C, Metges CC (2010) Foetal umbilical venous and arterial plasma amino acid concentrations are depending on the protein level of gestation diets fed to gilts. In: Matteo Corvetto G (ed) Energy and protein metabolism and nutrition. EAAP publication No. 127, 3rd EAAP ISEP, Parma, Italy, 6–10 Sept 2010, The Netherlands, pp 221–223
Görs S, Lang IS, Junghans P, Hennig U, Stabenow B, Schneider F, Otten W, Rehfeldt C, Metges CC (2009) Influence of maternal low and high protein diets during pregnancy in young sows on glucose metabolism of their offspring. Proc Soc Nutr Physiol 18 (Abstr.)
Kanitz E, Gräbner M, Tuchscherer M, Brüssow KP, Stabenow B, Rehfeldt C, Metges CC, Otten W (2010) An inadequate maternal protein diet during pregnancy in pigs alters the expression of corticosteroid receptors and 11ß-hydroxysteroid dehydrogenase isoforms in the placenta and fetal brain. Endocr Abstr 22:P261
Gregoire FM, Smas CM, Sul HS (1998) Understanding adipocyte differentiation. Physiol Rev 78:783–809
Bieswal F, Hay SM, McKinnon C, Reusens B, Cuignet M, Rees WD, Remacle C (2004) Prenatal protein restriction does not affect the proliferation and differentiation of rat preadipocytes. J Nutr 134:1493–1499
Sarr O, Louveau I, Kalbe C, Metges CC, Rehfeldt C, Gondret F (2010) Prenatal exposure to maternal low or high protein diets induces modest changes in the adipose tissue proteome of newborn piglets. J Anim Sci 88:1626–1641
Louveau I, Gondret F (2004) Regulation of development and metabolism of adipose tissue by growth hormone and the insulin-like growth factor system. Domest Anim Endocrinol 27:241–255
Kalbe C, Rehfeldt C (2005) Aspects of prenatal development of muscle and adipose tissue: principles, regulation, and influence of maternal nutrition. Berl Munch Tierarztl Wochenschr 118:509–520
Wigmore PM, Stickland NC (1983) Muscle development in large and small pig fetuses. J Anat 137:235–245
Lefaucheur L, Edom F, Ecolan P, Butlerbrowne GS (1995) Pattern of muscle-fiber type formation in the pig. Dev Dyn 203:27–41
Rehfeldt C, Kalbe C, Block J, Nürnberg G, Stabenow B, Metges CC (2008) Long-term effects of low and high protein feeding to pregnant sows on offspring at market weight. In: Proceedings of international congress of meat science & technology. 10–15 Aug 2008, Cape Town, South Africa, 7B.15
Bonen A (2010) Muscles as molecular and metabolic machines. Am J Physiol Endocrinol Metab 299:E143–E144
Lefaucheur L, Hoffman R, Okamura C, Gerrard D, Leger JJ, Rubinstein N, Kelly A (1997) Transitory expression of alpha cardiac myosin heavy chain in a subpopulation of secondary generation muscle fibers in the pig. Dev Dyn 210:106–116
Langley-Evans SC, Gardner DS, Jackson AA (1996) Maternal protein restriction influences the programming of the rat hypothalamic-pituitary-adrenal axis. J Nutr 126:1578–1585
Edwards CR, Benediktsson R, Lindsay RS, Seckl JR (1996) 11 beta-Hydroxysteroid dehydrogenases: key enzymes in determining tissue-specific glucocorticoid effects. Steroids 61:263–269
Kayali AG, Young VR, Goodman MN (1987) Sensitivity of myofibrillar proteins to glucocorticoid-induced muscle proteolysis. Am J Physiol 252:E621–E626
Millward DJ, Garlick PJ, Nnanyelugo DO, Waterlow JC (1976) The relative importance of muscle protein synthesis and breakdown in the regulation of muscle mass. Biochem J 156:185–188
Colenbrander B, de Jong FH, Wensing CJ (1978) Changes in serum testosterone concentrations in the male pig during development. J Reprod Fertil 53:377–380
Mayer M, Rosen F (1977) Interaction of glucocorticoids and androgens with skeletal muscle. Metabolism 26:937–962
Zhao W, Pan J, Zhao Z, Wu Y, Bauman WA, Cardozo CP (2008) Testosterone protects against dexamethasone-induced muscle atrophy, protein degradation and MAFbx upregulation. J Steroid Biochem Mol Biol 110:125–129
Graebner M, Kanitz E, Tuchscherer M, Stabenow B, Metges CC, Rehfeldt C, Otten W (2009) Maternal low and high protein diets during pregnancy affect body weight and stress reactivity in the offspring of pigs. J Anim Sci 87 E-Suppl 2/J Dairy Sci 92 E-Suppl. 1:234 (Abstr.)
Florini JR, Magri KA, Ewton DZ, James PL, Grindstaff K, Rotwein PS (1991) “Spontaneous” differentiation of skeletal myoblasts is dependent upon autocrine secretion of insulin-like growth factor-II. J Biol Chem 266:15917–15923
Florini JR, Ewton DZ, Coolican SA (1996) Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev 17:481–517
Kalbe C, Mau M, Rehfeldt C (2008) Developmental changes and the impact of isoflavones on mRNA expression of IGF-I receptor, EGF receptor and related growth factors in porcine skeletal muscle cell cultures. Growth Horm IGF Res 18:424–433
Lee CY, Chung CS, Simmen FA (1993) Ontogeny of the porcine insulin-like growth factor system. Mol Cell Endocrinol 93:71–80
Peng M, Pelletier G, Palin MF, Veronneau S, LeBel D, Abribat T (1998) Ontogeny of IGFs and IGFBPs mRNA levels and tissue concentrations in liver, kidney and skeletal muscle of pig. Growth Dev Aging 60:171–187
Gerrard DE, Okamura CS, Ranalletta MA, Grant AL (1998) Developmental expression and location of IGF-I and IGF-II mRNA and protein in skeletal muscle. J Anim Sci 76:1004–1011
Fix JS, Cassady JP, Herring WO, Holl JW, Culbertson MS, See MT (2010) Effect of piglet birth weight on body weight, growth, backfat, and longissimus muscle area of commercial market swine. Livest Sci 127:51–59
Beaulieu AD, Aalhus JL, Williams NH, Patience JF (2010) Impact of piglet birth weight, birth order, and litter size on subsequent growth performance, carcass quality, muscle composition, and eating quality of pork. J Anim Sci 88:2767–2778
Bieswal F, Ahn MT, Reusens B, Holvoet P, Raes M, Rees WD, Remacle C (2006) The importance of catch-up growth after early malnutrition for the programming of obesity in male rat. Obesity (Silver Spring) 14:1330–1343
Bol VV, Delattre AI, Reusens B, Raes M, Remacle C (2009) Forced catch-up growth after fetal protein restriction alters the adipose tissue gene expression program leading to obesity in adult mice. Am J Physiol Regul Integr Comp Physiol 297:R291–R299
Liang J, Zhang X, Zhao R, Maak S, Yang X (2010) Effect of maternal protein restriction on lipid metabolism in Meishan piglets at weaning. Livest Sci 136:157–163
Snoeck A, Remacle C, Reusens B, Hoet JJ (1990) Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Biol Neonate 57:107–118
Bennis-Taleb N, Remacle C, Hoet JJ, Reusens B (1999) A low-protein isocaloric diet during gestation affects brain development and alters permanently cerebral cortex blood vessels in rat offspring. J Nutr 129:1613–1619
Acknowledgments
This study was supported by a grant of the Deutsche Forschungsgemeinschaft (DFG), Bonn, to CK and CR (KA 1844/1-1; PAK24). The authors express their gratitude to H. Sievert and colleagues as well as Dr. vet. med. O. Bellmann for expert animal care, to Dr. U. Hennig for diet preparation, and M. Jugert-Lund, A. Steinborn, M. Günther, H. Brandt, G. Nehring, and I. Grünwald (FBN Dummerstorf) and P. Ecolan (INRA Saint-Gilles) for assistance with tissue sampling and laboratory analysis. Thanks also to Dr. S. Görs, I. S. Lang, S. Dwars, I. Brüning, and K. Karpati for tissue sampling.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rehfeldt, C., Lefaucheur, L., Block, J. et al. Limited and excess protein intake of pregnant gilts differently affects body composition and cellularity of skeletal muscle and subcutaneous adipose tissue of newborn and weanling piglets. Eur J Nutr 51, 151–165 (2012). https://doi.org/10.1007/s00394-011-0201-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-011-0201-8