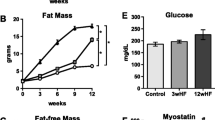
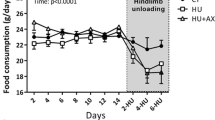
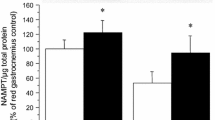
Abstract
Purpose
Decrease in activity stress induces skeletal muscle atrophy. A previous study showed that treatment with a high level (20%) of isoflavone inhibits muscle atrophy after short-term denervation (at 4 days) in mice. The present study was designed to elucidate whether the dietary isoflavone aglycone (AglyMax) at a 0.6% prevents denervation-mediated muscle atrophy, based on the modulation of atrogin-1- or apoptosis-dependent signaling.
Methods
Mice were fed either a normal diet or 0.6% AglyMax diet. One week later, the right sciatic nerve was cut. The wet weight, mean fiber area, amount of atrogin-1 and cleaved caspase-3 proteins, and the percentages of apoptotic nuclei were examined in the gastrocnemius muscle at 14 days after denervation.
Results
The 0.6% AglyMax diet significantly attenuated denervation-induced decreases in fiber atrophy but not the muscle wet weight. In addition, dietary isoflavone suppressed the denervation-induced apoptosis in spite of there being no significant changes in the amount of cleaved caspase-3 protein. In contrast, the 0.6% AglyMax diet did not significantly modulate the protein expression of atrogin-1 in the denervated muscle of mice.
Conclusions
The isoflavone aglycone (AglyMax) at a 0.6% significantly would modulate muscle atrophy after denervation in mice, probably due to the decrease in apoptosis-dependent signaling.





Similar content being viewed by others
References
Sakuma K, Yamaguchi A (2015) Sarcopenia and its intervention. In: Yu BP (ed) Nutrition, exercise and epigenetics: ageing interventions. Springer, Berlin, pp 127–152
Sakuma K, Yamaguchi A (2010) Molecular mechanisms in aging and current strategies to counteract sarcopenia. Curr Aging Sci 3(2):90–101
Camerino GM, Desaphy JF, De Bellis M, Capogrosso RF, Cozzoli A, Dinardo MM, Caloiero R, Musaraj K, Fonzino A, Conte E, Jagerschmidt C, Namour F, Liantonio A, De Luca A, Conte Camerino D, Pierno S (2015) Effects of nandrolone in the counteraction of skeletal muscle atrophy in a mouse model of muscle disuse: molecular biology and functional evaluation. PLoS One 10(6):e0129686
Qin W, Pan J, Wu Y, Bauman WA, Cardozo C (2015) Anabolic steroids activate calcineurin-NFAT signaling and thereby increase myotube size and reduce denervation atrophy. Mol Cell Endocrinol 399:336–345
Becker C, Lord SR, Studenski SA, Warden SJ, Fielding RA, Recknor CP, Hochberg MC, Ferrari SL, Blain H, Binder EF, Rolland Y, Poiraudeau S, Benson CT, Myers SL, Hu L, Ahmad QI, Pacuch KR, Gomez EV, Benichou O, STEADY Group (2015) Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol 3(12):948–957
Phillips SM (2015) Nutritional supplements in support of resistance exercise to counter age-related sarcopenia. Adv Nutr 6(4):452–460
Sakuma K, Yamaguchi A (2015) An overview of the therapeuticc strategies for preventing sarcopenia. In: Sakuma K (ed) Basic biology and current understanding of skeletal muscle. Nova Science Publishers, Hauppauge, pp 87–122
Thomas DK, Quinn MA, Saunders DH, Creig CA (2016) Protein supplementation does not significantly augment the effects of resistance exercise training in older adults: a systematic review. J Am Med Dir Assoc 17(10):959.e1–959.e9
Tham DM, Gardner CD, Haskell WL (1998) Clinical review 97: potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. J Clin Endocrinol Metab 83(7):2223–2235
Beekmann K, de Haan LH, Actis GL, Houtman R, van Bladeren PJ, Rietjens IM (2015) The effect of glucuronidation on isoflavone induced estrogen receptor (ER) α and ERβ mediated coregulator interactions. J Steroid Biochem Mol Biol 154:245–253
Kurrat A, Blei T, Kluxen FM, Mueller DR, Piechotta M, Soukup ST, Kulling SE, Diel P (2015) Lifelong exposure to dietary isoflavones reduces risk of obesity in ovariectomized Wistar rats. Mol Nutr Food Res 59(12):2407–2418
Abe T, Kohno S, Yama T, Ochi A, Suto T, Hirasaka K, Ohno A, Teshima-Kondo S, Okumura Y, Oarada M, Choi I, Mukai R, Terao J, Nikawa T (2013) Soy glycinin contains a functional inhibitory sequence against muscle-atrophy-associated ubiquitin ligase Cbl-b. Int J Endocrinol 2013:907565
Reagan-Shaw S, Nihal M, Ahmad N (2008) Does translation from animals to human studies revisited. FASEB J 22(3):659–661
Bloedon LT, Jeffcoat AR, Lopaczynski W, Schell MJ, Black TM, Dix KJ, Thomas BF, Albright C, Busby MG, Crowell JA, Zeisel SH (2002) Safety and pharmacyokinetics of purifyied soy isoflavones: single-dose administration to postmenopausal women. Am J Clin Nutr 76(5):1126–1137
Sakuma K, Akiho M, Nakashima H, Nakao R, Hirata M, Inashima S, Yamaguchi A, Yasuhara M (2008) Cyclosporin A modulates cellular localization of MEF2C protein and blocks fiber hypertrophy in the overloaded soleus muscle of mice. Acta Neuropathol 115(6):663–674
Akiho M, Nakashima H, Sakata M, Yamasa Y, Yamaguchi A, Sakuma K (2010) Expression profile of Notch-1 in mechanically overloaded plantaris muscle of mice. Life Sci 86(1–2):59–65
Henriques A, Croixmarie V, Priestman DA, Rosenbohm A, Dirrig-Grosch S, D’Ambra E, Huebecker M, Hussain G, Boursier-Neyret C, Echaniz-Laguna A, Ludolph AC, Platt FM, Walther B, Spedding M, Loeffler JP, Gonzalez De Aguilar JL (2015) Amyotrophic lateral sclerosis and denervation alter sphingolipids and up-regulate glucosylceramide synthase. Hum Mol Genet 24(25):7390–7405
Wall BT, Dirks ML, Snijders T, Stephens FB, Senden JM, Verscheijden ML, van Loon LJ (2015) Short-term muscle disuse atrophy is not associated with increased intramuscular lipid deposition or a decline in the maximal activity of key mitochondrial enzymes in young and older males. Exp Gerontol 61:76–83
Palacios-González B, Zarain-Herzberg A, Flores-Galicia I, Noriega LG, Alemán-Escondrillas G, Zarinan T, Ulloa-Aguirre A, Torres N, Tovar AR (2014) Genistein stimulates fatty acid oxidation in a leptin receptor-independent manner through the JAK2-mediated phosphorylation and activation of AMPK in skeletal muscle. Biochim Biophys Acta 1841(1):132–140
Wei JH, Chang NC, Chen SP, Geraldine P, Jayakumar T, Fong TH (2015) Comparative decline of the protein profiles of nebulin in response to denervation in skeletal muscle. Biochem Biophys Res Commun 466(1):95–102
MacDonald EM, Andres-Mateos E, Mejias R, Simmers JL, Mi R, Park JS, Ying S, Hoke A, Lee SJ, Cohn RD (2014) Denervation atrophy is independent from Akt and mTOR activation and is not rescued by myostatin inhibition. Dis Model Mech 7(4):471–481
Sandri M, Barberi L, Bijlsma AY, Blaauw B, Dyar KA, Milan G, Mammucari C, Meskers CG, Pallafacchina G, Paoli A, Pion D, Roceri M, Romanello V, Serrano AL, Toniolo L, Larsson L, Maier AB, Muñoz-Cánoves P, Musarò A, Pende M, Reggiani C, Rizzuto R, Schiaffino S (2013) Signaling pathways regulating muscle mass in ageing skeletal muscle. The role of IGF-1-Akt-mTOR-FoxO pathway. Biogerontology 14(3):303–323
Hwee DT, Baehr LM, Philp A, Baar K, Bodinne SC (2014) Maintenance of muscle mass and load-induced growth in muscle RING finger 1 null mice with age. Aging Cell 13(1):92–101
Bodine SC, Latres E, Baumhueter S, Lai VK-M, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan Z-Q, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294(5547):1704–1708
Bodine SC, Baehr LM (2014) Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab 307(6):E469–E484
Sun H, Qiu J, Chen Y, Yu M, Ding F, Gu X (2014) Proteomic and bioinformatic analysis of differentially expressed proteins in denervated skeletal muscle. Int J Mol Med 33(6):1586–1596
Alamdari N, Aversa Z, Castillero E, Gurav A, Petkova V, Tizio S, Hasselgren PO (2012) Resveratrol prevents dexamethasone-induced expression of the muscle atrophy-related ubiquitin ligases atrogin-1 and MuRF1 in cultured myotubes through a SIRT1-dependent mechanism. Biochem Biophys Res Commun 417(1):528–533
Sin TK, Yung BY, Yip SP, Chan LW, Wong CS, Tam EW, Siu PM (2015) SIRT1-dependent myoprotective effects of resveratrol on muscle injury induced by compression. Front Physiol 6:293
Le NH, Kim CS, Park T, Park JH, Sung MK, Lee DG, Hong SM, Choe SY, Goto T, Kawada T, Yu R (2014) Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediat Inflamm 2014:834294
Siu PM, Always SE (2009) Response and adaptation of skeletal muscle to denervation stress: the role of apoptosis in muscle loss. Front Biosci 14:432–452
Siu PM, Always SE (2005) Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. J Physiol 565(Pt 1):309–323
Hopkins D, Manchester KL, Gregory M (1983) Histochemical and biochemical characteristics of the transient hypertrophy of the denervated rat hemidiaphragm. Exp Neurol 81(2):279–293
Connor EA, McMahan UJ (1987) Cell accumulation in the junctional region of denervated muscle. J Cell Biol 104(1):109–120
Damarla M, Parniani AR, Johnston L (2014) Mitogen-activated protein kinase-activated protein kinase 2 mediates apoptosis during lung vascular permeability by regulating movement of cleaved caspase 3. Am J Respirat Cell Mol Biol 50(5):932–941
Messina S, Bitto A, Vita GL, Aguennouz M, Irrera N, Licata N, Sframeli M, Bruschetta D, Minutoli L, Altavilla D, Vita G, Squadrito F (2015) Modulation of neuronal nitric oxide synthase and apoptosis by the isoflavone genistein in mdx mice. Biofactors 41(5):324–329
Cea LA, Cisterna BA, Puebla C, Frank M, Figueroa XF, Cardozo C, Willecke K, Latorre R, Sáez JC (2013) De novo expression of connexin hemichannels in denervated fast skeletal muscles leads to atrophy. Proc Natl Acad Sci USA 110(40):16229–16234
Acknowledgements
This work was supported by a research Grant-in-Aid for Scientific Research C (No. 17K01755) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kunihiro Sakuma and all the co-authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tabata, S., Aizawa, M., Kinoshita, M. et al. The influence of isoflavone for denervation-induced muscle atrophy. Eur J Nutr 58, 291–300 (2019). https://doi.org/10.1007/s00394-017-1593-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1593-x