Abstract
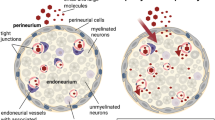
The molecular determinants and mechanisms involved in leukocyte trafficking across the blood–nerve barrier (BNB) in the acute inflammatory demyelinating polyradiculoneuropathy (AIDP) variant of Guillain–Barré syndrome are incompletely understood. Prior work using a flow-dependent in vitro human BNB model demonstrated a crucial role for αM-integrin (CD11b)-intercellular adhesion molecule-1 interactions in AIDP patient leukocyte trafficking. The aim of this study is to directly investigate the biological relevance of CD11b in AIDP pathogenesis. Immunohistochemistry was performed on three AIDP patient sural nerve biopsies to evaluate endoneurial leukocyte CD11b expression. A severe murine experimental autoimmune neuritis (sm-EAN) model was utilized to determine the functional role of CD11b in leukocyte trafficking in vivo and determine its effect on neurobehavioral measures of disease severity, electrophysiological assessments of axonal integrity and myelination and histopathological measures of peripheral nerve inflammatory demyelination. Time-lapse video microscopy and electron microscopy were employed to observe structural alterations at the BNB during AIDP patient leukocyte trafficking in vitro and in situ, respectively. Large clusters of endoneurial CD11b+ leukocytes associated with demyelinating axons were observed in AIDP patient sural nerves. Leukocyte CD11b expression was upregulated during sm-EAN. 5 mg/kg of a function-neutralizing monoclonal rat anti-mouse CD11b antibody administered after sm-EAN disease onset significantly ameliorated disease severity, as well as electrophysiological and histopathological parameters of inflammatory demyelination compared to vehicle- and isotype antibody-treated mice. Consistent with in vitro observations of leukocyte trafficking at the BNB, electron micrographs of AIDP patient sural nerves demonstrated intact electron-dense endoneurial microvascular intercellular junctions during paracellular mononuclear leukocyte transmigration. Our data support a crucial pathogenic role of CD11b in AIDP leukocyte trafficking, providing a potential therapeutic target for demyelinating variants of Guillain–Barré syndrome.







Similar content being viewed by others
References
Abe M, Sano Y, Maeda T, Shimizu F, Kashiwamura Y, Haruki H, Saito K, Tasaki A, Kawai M, Terasaki T, Kanda T (2012) Establishment and characterization of human peripheral nerve microvascular endothelial cell lines: a new in vitro blood–nerve barrier (BNB) model. Cell Struct Funct 37:89–100
Bianchi E, Molteni R, Pardi R, Dubini G (2013) Microfluidics for in vitro biomimetic shear stress-dependent leukocyte adhesion assays. J Biomech 46:276–283. doi:10.1016/j.jbiomech.2012.10.024
Chiang S, Ubogu EE (2013) The role of chemokines in Guillain–Barre syndrome. Muscle Nerve 48:320–330. doi:10.1002/mus.23829
Creange A, Chazaud B, Sharshar T, Plonquet A, Poron F, Eliezer MC, Raphael JC, Gherardi RK (2001) Inhibition of the adhesion step of leukodiapedesis: a critical event in the recovery of Guillain–Barre syndrome associated with accumulation of proteolytically active lymphocytes in blood. J Neuroimmunol 114:188–196
Dalakas MC (2015) Pathogenesis of immune-mediated neuropathies. Biochim Biophys Acta 1852:658–666. doi:10.1016/j.bbadis.2014.06.013
Golde WT, Gollobin P, Rodriguez LL (2005) A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 34:39–43. doi:10.1038/laban1005-39
Greathouse KM, Palladino SP, Dong C, Helton ES, Ubogu EE (2016) Modeling leukocyte trafficking at the human blood–nerve barrier in vitro and in vivo geared towards targeted molecular therapies for peripheral neuroinflammation. J Neuroinflam 13:3. doi:10.1186/s12974-015-0469-3
Hadden RD, Karch H, Hartung HP, Zielasek J, Weissbrich B, Schubert J, Weishaupt A, Cornblath DR, Swan AV, Hughes RA, Toyka KV, Plasma Exchange/Sandoglobulin Guillain–Barre Syndrome Trial G (2001) Preceding infections, immune factors, and outcome in Guillain–Barre syndrome. Neurology 56:758–765
Kanda T (2013) Biology of the blood–nerve barrier and its alteration in immune mediated neuropathies. J Neurol Neurosurg Psychiatry 84:208–212. doi:10.1136/jnnp-2012-302312
Kiefer R, Kieseier BC, Stoll G, Hartung HP (2001) The role of macrophages in immune-mediated damage to the peripheral nervous system. Prog Neurobiol 64:109–127
Kieseier BC, Kiefer R, Gold R, Hemmer B, Willison HJ, Hartung HP (2004) Advances in understanding and treatment of immune-mediated disorders of the peripheral nervous system. Muscle Nerve 30:131–156. doi:10.1002/mus.20076
Kieseier BC, Tani M, Mahad D, Oka N, Ho T, Woodroofe N, Griffin JW, Toyka KV, Ransohoff RM, Hartung HP (2002) Chemokines and chemokine receptors in inflammatory demyelinating neuropathies: a central role for IP-10. Brain 125:823–834
Man S, Ubogu EE, Ransohoff RM (2007) Inflammatory cell migration into the central nervous system: a few new twists on an old tale. Brain Pathol 17:243–250. doi:10.1111/j.1750-3639.2007.00067.x
zu Horste GM, Hartung HP, Kieseier BC (2007) From bench to bedside—experimental rationale for immune-specific therapies in the inflamed peripheral nerve. Nat Clin Pract Neurol 3:198–211. doi:10.1038/ncpneuro0452
Mizisin AP, Weerasuriya A (2011) Homeostatic regulation of the endoneurial microenvironment during development, aging and in response to trauma, disease and toxic insult. Acta Neuropathol 121:291–312. doi:10.1007/s00401-010-0783-x
Muller WA (2003) Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol 24:327–334
Muller WA (2011) Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol 6:323–344. doi:10.1146/annurev-pathol-011110-130224
Musso AM, Zanusso GL, Bonazzi ML, Tomelleri G, Bonetti B, Moretto G, Vio M, Monaco S (1994) Increased serum levels of ICAM-1, ELAM-1 and TNF-alpha in inflammatory disorders of the peripheral nervous system. Ital J Neurol Sci 15:267–271
Orlikowski D, Chazaud B, Plonquet A, Poron F, Sharshar T, Maison P, Raphael JC, Gherardi RK, Creange A (2003) Monocyte chemoattractant protein 1 and chemokine receptor CCR2 productions in Guillain–Barre syndrome and experimental autoimmune neuritis. J Neuroimmunol 134:118–127
Pollard JD, Baverstock J, McLeod JG (1987) Class II antigen expression and inflammatory cells in the Guillain–Barre syndrome. Ann Neurol 21:337–341. doi:10.1002/ana.410210404
Putzu GA, Figarella-Branger D, Bouvier-Labit C, Liprandi A, Bianco N, Pellissier JF (2000) Immunohistochemical localization of cytokines, C5b-9 and ICAM-1 in peripheral nerve of Guillain–Barre syndrome. J Neurol Sci 174:16–21
Plasma Exchange/Sandoglobulin Guillain–Barre Syndrome Trial Group (1997) Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain–Barre syndrome. Lancet 349:225–230
Rinaldi S (2013) Update on Guillain–Barre syndrome. J Peripher Nerv Syst 18:99–112. doi:10.1111/jns5.12020
Schmidt B, Toyka KV, Kiefer R, Full J, Hartung HP, Pollard J (1996) Inflammatory infiltrates in sural nerve biopsies in Guillain–Barre syndrome and chronic inflammatory demyelinating neuropathy. Muscle Nerve 19:474–487. doi:10.1002/(SICI)1097-4598(199604)19:4<474:AID-MUS8>3.0.CO;2-9
Simon SI, Green CE (2005) Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng 7:151–185. doi:10.1146/annurev.bioeng.7.060804.100423
Ubogu EE (2013) Chemokine-dependent signaling pathways in the peripheral nervous system. Methods Mol Biol 1013:17–30. doi:10.1007/978-1-62703-426-5_2
Ubogu EE (2013) The molecular and biophysical characterization of the human blood–nerve barrier: current concepts. J Vasc Res 50:289–303. doi:10.1159/000353293
Ubogu EE (2015) Inflammatory neuropathies: pathology, molecular markers and targets for specific therapeutic intervention. Acta Neuropathol 130:445–468. doi:10.1007/s00401-015-1466-4
Ubogu EE, Callahan MK, Tucky BH, Ransohoff RM (2006) Determinants of CCL5-driven mononuclear cell migration across the blood-brain barrier. Implications for therapeutically modulating neuroinflammation. J Neuroimmunol 179:132–144. doi:10.1016/j.jneuroim.2006.06.004
Ubogu EE, Yosef N, Xia RH, Sheikh KA (2012) Behavioral, electrophysiological, and histopathological characterization of a severe murine chronic demyelinating polyneuritis model. J Peripher Nerv Syst 17:53–61. doi:10.1111/j.1529-8027.2012.00375.x
van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA (2014) Guillain–Barre syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol 10:469–482. doi:10.1038/nrneurol.2014.121
Willison HJ, Jacobs BC, van Doorn PA (2016) Guillain–Barre syndrome. Lancet. doi:10.1016/S0140-6736(16)00339-1
Winger RC, Koblinski JE, Kanda T, Ransohoff RM, Muller WA (2014) Rapid remodeling of tight junctions during paracellular diapedesis in a human model of the blood-brain barrier. J Immunol 193:2427–2437. doi:10.4049/jimmunol.1400700
Xia RH, Yosef N, Ubogu EE (2010) Clinical, electrophysiological and pathologic correlations in a severe murine experimental autoimmune neuritis model of Guillain–Barre syndrome. J Neuroimmunol 219:54–63. doi:10.1016/j.jneuroim.2009.11.019
Xia RH, Yosef N, Ubogu EE (2010) Dorsal caudal tail and sciatic motor nerve conduction studies in adult mice: technical aspects and normative data. Muscle Nerve 41:850–856. doi:10.1002/mus.21588
Yonekawa K, Harlan JM (2005) Targeting leukocyte integrins in human diseases. J Leukoc Biol 77:129–140. doi:10.1189/jlb.0804460
Yosef N, Ubogu EE (2012) Alpha(M)beta(2)-integrin-intercellular adhesion molecule-1 interactions drive the flow-dependent trafficking of Guillain–Barre syndrome patient derived mononuclear leukocytes at the blood–nerve barrier in vitro. J Cell Physiol 227:3857–3875. doi:10.1002/jcp.24100
Yosef N, Xia RH, Ubogu EE (2010) Development and characterization of a novel human in vitro blood–nerve barrier model using primary endoneurial endothelial cells. J Neuropathol Exp Neurol 69:82–97. doi:10.1097/NEN.0b013e3181c84a9a
Yuan F, Yosef N, Lakshmana Reddy C, Huang A, Chiang SC, Tithi HR, Ubogu EE (2014) CCR2 gene deletion and pharmacologic blockade ameliorate a severe murine experimental autoimmune neuritis model of Guillain–Barre syndrome. PLoS One 9:e90463. doi:10.1371/journal.pone.0090463
Yuki N, Hartung HP (2012) Guillain–Barre syndrome. N Engl J Med 366:2294–2304. doi:10.1056/NEJMra1114525
Zhang HL, Zheng XY, Zhu J (2013) Th1/Th2/Th17/Treg cytokines in Guillain–Barre syndrome and experimental autoimmune neuritis. Cytokine Growth Factor Rev 24:443–453. doi:10.1016/j.cytogfr.2013.05.005
Acknowledgments
Special thanks to past and current employees of the Shin J. Oh Muscle and Nerve Histopathology Laboratory, University of Alabama at Birmingham for archived peripheral nerves and processed slides from AIDP patients and controls. Special thanks to EMLABS Inc. for performing the electron microscopy work. This project was supported by National Institutes of Health (NIH) Grant R21 NS078226 (2012-2015) and institutional funds from the University of Alabama at Birmingham. The funding sources had no involvement in the conduct of the research, manuscript preparation, data collection/analyses or decision to submit this work for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E.E.U. has received royalties from Baylor Licensing Group for simian virus-40 large T-antigen immortalized human endoneurial endothelial cells and from Springer Science + Business Media for an edited book on laboratory protocols that describes the flow-dependent in vitro BNB assay. C.D., S.P.P. and E.S.H. have nothing to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2016_1599_MOESM1_ESM.mp4
Supplementary Video 1. AIDP patient leukocyte trafficking at the in vitro blood–nerve barrier. A compressed two minute video generated via real-time time-lapse digital microscopy demonstrates leukocytes from an untreated AIDP patient (phase bright) rolling and undergoing arrest and firm adhesion between cytokine stimulated confluent BNB-forming endoneurial endothelial cells (dark spindle-shaped background cells) under a continuous flow velocity of 1 mm/s in vitro. Diapedesis, detected by a change in leukocyte phase from bright to dark, was observed via the paracellular route with slight endothelial cell displacement, similar to in situ ultrastructural observations in AIDP patient sural nerve biopsies. Leukocytes were attracted to sites of firm adhesion and active transmigration, consistent with inflammatory cell clusters seen in AIDP patient biopsies. Frame rate = 10 frames per second (10X normal, compressing 20 minutes in real time) (MP4 7568 kb)
Rights and permissions
About this article
Cite this article
Dong, C., Palladino, S.P., Helton, E.S. et al. The pathogenic relevance of αM-integrin in Guillain–Barré syndrome. Acta Neuropathol 132, 739–752 (2016). https://doi.org/10.1007/s00401-016-1599-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-016-1599-0