Abstract
Matrix metalloproteinase-9 (MMP-9) appears to be a major protease responsible for the degradation of matrix and growth-promoting agents in chronic wounds. Honey has been successfully used for treating non-healing wounds associated with infections. However, the mechanisms of its action at the cellular level have remained poorly understood. The aim of this study was to investigate the effect of fir honeydew honey on TNF-α-induced MMP-9 expression and secretion from human keratinocytes (HaCaT) and to identify the honey component(s) responsible for a discovered effect. A C18 solid-phase column was used for preparation of honey aqueous extract (HAE). Expression and production of MMP-9 by HaCaT cells were determined by reverse transcription-PCR, gelatine zymography and Western blot analysis using a polyclonal antibody against MMP-9. We found that HAE inhibited TNF-α-induced production of MMP-9 in keratinocytes in a dose-dependent manner at both the mRNA and protein levels. Apigenin and kaempferol, identified flavonoids in HAE, markedly inhibited MMP-9 production from HaCaT and epidermal keratinocytes. Taken together, fir honeydew honey, which contains certain flavonoids, prevents TNF-α-induced proteolytic activity in cutaneous inflammation. Thus, our findings provide clear evidence that honey may serve as a natural treatment for dermatological problems associated with a persistent inflammation.
Similar content being viewed by others
Introduction
Exaggerated and uncontrolled inflammation is a common factor that contributes to extracellular matrix (ECM) destruction, cellular senescence and non-healing in cutaneous wounds. Many theories have been devised to explain the profound inflammatory dysregulation and various possible contributing factors, such as pro-inflammatory cytokines, growth factors and matrix metalloproteinases (MMPs), have been identified [16].
One of the major consequences of the persistent inflammatory response in the wound is an unbalanced proteolytic activity, which overwhelms the protective mechanisms of a local tissue [2, 36]. Indeed, higher levels of proteolytic activity were found in patients with chronic wounds than in those with acute wounds [31, 34]. The MMPs, a dominant protease group in inflammation and the wound healing process (reviewed in [36]), are a large family of zinc-dependent endopeptidases capable of degrading ECM components [25]. The expression and the activity of various MMPs, particularly MMP-9, are highly up-regulated in chronic wounds [28]. Moreover, pro-inflammatory cytokines, potent inducers of MMP expression in chronic wounds, have been shown to down-regulate the expression of tissue inhibitors of MMPs [4].
MMP-9, an inducible gelatinase secreted by several cell types including stressed keratinocytes, plays an important role in normal wound healing particularly related to ECM remodelling and re-epithelialisation [12, 20]. Here, its ability to cleave collagen type IV into small peptides is pivotal. It was found that MMP-9 is highly but transiently expressed in newly wounded tissue [29]. On the other hand, in chronic wounds, the level of MMP-9 remains permanently high and MMP-9 appears to be a major protease responsible for the degradation of matrix and cell growth-promoting agents in chronic wound fluids [23]. The synthesis and secretion of MMP-9 can be stimulated by various stimuli including TNF-α during inflammation [19]. The function of MMP-9 is controlled by local concentration of specific inhibitor known as tissue inhibitor of MMP-9, TIMP-1. The MMP-9/TIMP-1 ratio was shown to be inversely correlated with healing—elevated levels of MMP-9 predict poor healing in pressure ulcers [13].
Promoting, returning to and maintaining a normal wound microenvironment can be difficult. Previous use of isolated molecules and compounds to modify the wound environment yielded ambiguous results [3, 24, 30]. There is an urgent need for new therapeutic agents that would counteract the destructive components in wound fluids. In addition, these therapeutic agents should ideally possess antibacterial, anti-biofilm and anti-inflammatory properties. Honey fulfils all of the above-mentioned requirements, and it has already been successfully used in chronic wound management [22].
It is generally assumed that the antibacterial properties of honey play a major role in a healing process of infected chronic wounds. Besides eliminating pathogens from wounds, honey also provides an appropriate moist environment for proper wound healing. Besides the direct antimicrobial effects of honey [11], research has also focused on identification of the substances responsible for its anti-inflammatory [5, 8, 35] and immunomodulatory effects [18, 27, 32]. Although several compounds with these effects were identified, subjacent mechanisms of action associated with the anti-inflammatory effects of honey in wound healing have not been fully elucidated.
In this work, we investigated the effects of honey on tumour necrosis factor-α (TNF-α)-induced MMP-9 expression and production from human keratinocytes (HaCaT) and identified the substances in fir honeydew honey responsible for such an effect.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), foetal calf serum (FCS), l-glutamine, antibiotics mixture (100 IU/ml penicillin and 100 μg/ml streptomycin) and trypsin-ethylenediaminetetraacetic acid (EDTA) were purchased from Biochrom AG (Germany). Rabbit polyclonal antibodies against human MMP-9 and tissue inhibitor of metalloproteinase-1 (TIMP-1) (Anti-TiMP-1, C-terminus) were purchased from Millipore (USA). Goat anti-rabbit horseradish peroxidise-conjugated secondary antibody was purchased from Promega (USA). Human recombinant TNF-α, phenolic acids [p-coumaric acid (C9008) and ferulic acid (12870-8)] and flavonoids [quercetin (Q0125), apigenin (A3145), kaempferol (K0133), isorhamnetin (17794), chrysin (C80105), naringenin (N5893) and galangin (282200)] were purchased from Sigma-Aldrich (Germany). The stock solutions of phenolic acids and flavonoids (100 mM) were prepared in dimethyl sulfoxide (DMSO). All other chemicals and reagents were obtained from Sigma-Aldrich (Germany) unless otherwise indicated. Fir honeydew honey samples were obtained from Mr. Jozef Volansky (Medar apiary, Bardejov, Slovakia).
Honey aqueous phenolic extract preparation
Honeydew honey sample (10 g) was fully dissolved in five parts (w/v) of distilled water (adjusted to pH 2 with HCl). This solution was applied to a Sep-Pack C18 cartridge (Waters, USA), which was pre-equilibrated by methanol (10 ml) followed by water (10 ml). The phenolic content was eluted with 80 % (v/v) methanol (2 ml). The methanolic extract was freeze-dried on a SpeedVac concentrator (Thermo Fisher Scientific, USA) and dissolved in distilled water. The honey aqueous extract (HAE) was aliquoted and stored at −20 °C. HAE was further analysed by high-performance liquid chromatography (HPLC) and ultra-performance liquid chromatography (UPLC) to identify of individual phenolic compounds.
Cell culture
Human HaCaT keratinocytes were purchased from Cell Lines Service (Germany). The HaCaT cells were cultured in DMEM supplemented with 10 % FCS, 2 mM glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin. The cells were sub-cultured every 4 days at 37 °C under 5 % CO2 atmosphere. For all experiments, cells were grown to 70–80 % confluence and incubated in serum-free DMEM 24 h prior to a treatment with HAE or phenolic compounds. Cultures were treated with HAE (0–2.5 mg/ml) or phenolic compounds (10–100 μM) or DMSO as vehicle for 24 h. The final concentration of DMSO in the culture medium was below 0.1 % (v/v). Cultures were then exposed to TNF-α (10 ng/ml) for an additional 24 h.
Human epidermal keratinocytes (HEK) (Life technologies, UK) were cultured in EpiLife® medium supplemented with human keratinocyte growth supplement (Life technologies, UK). HEK were sub-cultured according to manufacturer’s instructions and cultures were treated with individual phenolic compounds or DMSO as mentioned above.
Cell viability assay
The cytotoxic effect of HAE on HaCaT cells was measured by the Alamar Blue assay (Life Technologies, UK) according to the manufacturer’s protocol. Results were expressed as the percentage of cytotoxicity calculated according to the manufacturer’s equation.
Gelatin zymography assay
Conditioned media of HaCaT and HEK cell cultures were subjected to gelatin zymography as was previously described [10]. Briefly, non-reducing LDS sample buffer (Life Technologies, UK) was added to aliquots of culture medium supernatants at a ratio of 1:4. Twenty microlitre aliquots were separated on 8 % sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gels containing 0.5 mg/ml gelatine under non-reducing conditions. The gels were washed in 2.5 % Triton X-100 for 60 min at room temperature to remove the SDS and were subsequently incubated in a developer buffer [50 mM Tris (pH 7.8), 5 mM CaCl2 and 0.2 M NaCl] for 24 h at 37 °C. Gels were stained with 0.5 % Coomassie Brilliant Blue G-250 and the bands of proteolytic activity were quantified by densitometry (Quantity One, Bio-Rad, USA).
Western blot analysis
Western blot analysis was performed using the semi-dry blotting method [6]. Concentrated supernatants derived from HaCaT cells, cultured as described above, were prepared as follows: An initial volume of 0.5 ml of culture supernatant was collected from each well (control and treated cultures). Subsequently, a tenfold concentration was performed using ultracentrifugal filter devices (10,000 MWCO; Sartorius, Germany). Equal volumes (15 μl) of the concentrated supernatants were subjected to electrophoresis using 10 % SDS-PAGE gels. Proteins were transferred onto a nitrocellulose membrane and probed with the anti-MMP-9 and anti-TIMP-1 antibody diluted at 1:400 and 1:1,000 in blocking buffer, respectively. Detection was carried out using horseradish peroxidise-conjugated secondary antibodies. Visualisation of the immunoactive bands was performed using the enhanced chemiluminescence kit (Kodak, USA). Quantification was done by densitometry (Quantity One, Bio-Rad).
Reverse transcription-PCR (RT-PCR) assay
Total RNA was isolated using a GenElute Mammalian Total RNA Miniprep kit (Sigma-Aldrich) according to manufacturer’s instructions. Total RNA (1.5 μg) was reverse transcribed using the Super cDNA RT Kit (E. coli, Slovakia) according to manufacturer’s instructions. The transcribed cDNA was then amplified by PCR using the following primers for MMP-9 (sense: 5′-CACTGTCCACCCCTCAGAGC-3′, antisense: 5′-GCCACTTGTCGGCGATAAGG-3′; 243 bp) and β-actin (sense: 5′-GGACTTCGAGCAAGAGATGG-3′, antisense: 5′-AGCACTGTGTTGGCGTACAG-3′; 243 bp) as a control. PCR products were analysed on 2 % agarose gel and specific bands were quantified by densitometry (Quantity One, Bio-Rad).
Identification of phenolic compounds in HAE
HAEs were analysed on an Agilent 1100 HPLC system equipped with a photodiode-array detector and an ion-trap mass spectrometer detector in series (Agilent Technologies, Germany). Chromatographic separation was carried out on a reverse phase Pursuit XRs C18 column (Agilent Technologies, Germany) (250 × 4 mm, 5 μm particle size) using water with 1 % formic acid (A) and acetonitrile (B) as mobile phases. The gradient profile was: 0–20 min, 5–30 % B; 20–40 min, 30–70 % B; 40–45 min, 70–95 % B; 46–48 min, 95–5 % B and maintaining at 5 % for 55 min. A 20-μl sample was injected into the column operating at room temperature at a flow rate of 1 ml/min. Chromatograms were recorded at 280, 320, 340 and 360 nm wavelengths.
The HPLC system was coupled in series to an Esquire 1100 ion-trap mass spectrometer (IT) equipped with an electrospray interface (ESI) (Bruker, Germany). Nitrogen was used as a drying gas with a flow of 11 l/min and temperature of 350 °C and nebulising gas at a pressure of 448 kPa. The capillary voltage was set at 4 kV. Mass scan (MS) and daughter (MS–MS) spectra were recorded in negative mode in the range of 100–1,500 m/z with a target mass of 700. The analyses were performed in triplicate.
Compounds were identified according to their molecular weight (mass spectra), characteristic UV spectra, their MS/MS fragmentations and the wide information previously reported in the literature. Moreover, to confirm the identification of the compounds, samples were analysed with the Agilent 1290 Infinity UPLC system coupled to a 6550 Accurate-Mass QTOF (Agilent Technologies, Germany). QTOF MS (quadrupole time-of-flight mass spectrometry) provided a very high mass resolution that allows calculating the elemental composition of the compounds based on the mass accuracy and isotopic pattern.
Hydroxycinnamic acids were quantified using UV detection at 320 nm with the calibration curve obtained for caffeic acid, flavonols at 360 nm with the calibration curve of quercetin, flavanones at 280 nm with the calibration curve of naringenin and flavones at 340 nm with the calibration curve of apigenin, except for chrysin that was quantified with its own standard.
Statistical analysis
Results are presented as mean ± standard error of mean (SEM). All data were statistically analysed by one-way analysis of variance (ANOVA) and Bonferroni’s test to determine whether there were differences within groups. P values less than 0.05 were considered to be significant. Analyses were performed using GraphPad Prism (GraphPad Software, USA).
Results
Cytotoxic effect of HAE on HaCaT cells
Initially, we assessed the cytotoxicity of prepared HAE extract on HaCaT cells using the Alamar Blue assay. HAE had no cytotoxic effect on the cells at concentrations up to 2.5 mg/ml (Fig. 1). All subsequent experiments were carried out with less than 2.5 mg/ml HAE.
Effects of honey aqueous extract (HAE) on HaCaT cell viability. Cells were treated with the indicated concentrations of HAE for 48 h, and cell viability was determined by the Alamar Blue assay. Data are expressed as % of control and each column represents the mean ± SEM of independent four tests. Asterisks indicate a significant difference compared with the control group, *P < 0.01
Inhibitory effect of HAE on secreted MMP-9 in TNF-α-stimulated HaCaT cells
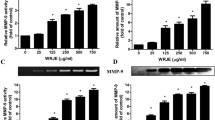
The effect of HAE on TNF-α-induced MMP-9 secretion was investigated by using a gelatine zymography assay. Treatment of HaCaT cells with TNF-α (10 ng/ml) for 24 h resulted in an increase in MMP-9 secretion to the extracellular milieu. TNF-α was found to markedly stimulate MMP-9 but not MMP-2 secretion (Fig. 2a). As shown further in Fig. 2a, treatment of the keratinocytes with HAE 24 h before and during the exposure to TNF-α for 24 h diminished MMP-9 secretion from the cells in a dose-dependent manner. At the highest HAE concentration (2.5 mg/ml), the activity of MMP-9 was reduced nearly to the level detected in non-TNF-α-treated control cells. Treatment of HaCaT cells with HAE (2.5 mg/ml) and without TNF-α did not show any changes in production of MMP-9 as compared to untreated cells (data not shown).
The effects of honey aqueous extract (HAE) on TNF-α-induced a MMP-9 production and proteolytic activity, b MMP-9 mRNA expression and c, d MMP-9 and TIMP-1 protein expression in HaCaT cells. HaCaT cells were treated with different doses of HAE for 24 h and then cells were exposed to TNF-α (10 ng/ml) for additional 24 h. a Conditioned equal volumes of the culture media were collected and subjected to gelatine zymography. Densitometric quantification of MMP-9 in culture media is presented. b The mRNA levels of MMP-9 and β-actin were determined by RT-PCR and quantified densitometrically. c, d Conditioned equal volumes of the concentrated culture media were subjected to 10 % SDS-PAGE gels. MMP-9 (92 kDa) and TIMP-1 (29 kDa) protein expression was determined by Western blotting and quantified densitometrically. Data are expressed as a mean with SEM of three independent measurements. Asterisks indicate a significant difference from the TNF-α-treated control group, *P < 0.001
HAE inhibits MMP-9 expression in HaCaT cells
Our results indicated the possibility that HAE regulates the expression of MMP-9 induced by TNF-α in keratinocytes. Therefore, we assessed the cell mRNA and protein levels of MMP-9 by RT-PCR and Western blot analysis, respectively. As shown in Fig. 2b and c, both mRNA and protein levels of MMP-9 were reduced by HAE treatment in a dose-dependent manner. At the highest HAE concentrations (2 and 2.5 mg/ml), MMP-9 mRNA and protein levels were reduced to the levels seen in non-TNF-α-treated control cells.
We also determined the effect of HAE on MMP-9-related endogenous inhibitor, TIMP-1. The protein levels of TIMP-1 were examined by Western blot analysis (Fig. 2d). Expression of TIMP-1 was significantly increased by HAE treatment in TNF-α-treated cells in concentrations ranging from 0.1 to 1 mg/ml compared to non-TNF-α-treated control cells. Interestingly, HAE treatment at a higher dose (2 and 2.5 mg/ml) reduced the production of TIMP-1 in TNF-α-stimulated cells and reduced the level of TIMP-1 to basal levels found in control cells.
Analysis of phenolic compounds in HAE
The main phenolic compounds identified in HAEs are summarised in Table 1. Seventeen compounds belonging to the groups of hydroxycinnamic acids (caffeic, coumaric and ferulic acid), flavonols (quercetin, kaempferol, galangin and their derivatives), flavones (apigenin, chrisoeriol and chrysin) and flavanones (naringenin, hesperetin and pinocembrin) were identified via the UV spectra and molecular formulas were obtained with high scores (>95 %) and low errors (<3 ppm). Some of these compounds have been further quantified by UV spectrometry determination at 320 nm (hydroxycinnamic acids), 360 nm (flavonols), 340 nm (flavanones) and 280 nm (flavones) (Table 1). The hydroxycinnamic acids, coumaric and ferulic acid were the most abundant compounds followed by naringenin and kaempferol. Naringenin 7-methyl ether, chrysin and pinocembrin were also found in high amounts. The chromatogram obtained at 360 nm with the quantified compounds is shown in Fig. 3a.
Characterisation of phenolic compounds from the honey aqueous extract (HAE) and determination of MMP-9 inhibitory activities. a HPLC chromatogram of HAE at 360 nm using the Pursuit XRs C18 column (Agilent Technologies) and water with 1 % formic acid and acetonitrile as the mobile phases. Numbers correspond to the main compounds detected and quantified that are reported in Table 1. b Effect of selected phenolic compounds at given concentrations on a reduction of TNF-α-induced MMP-9 production in HaCaT and human epidermal keratinocytes (HEK) cells. Equal volumes of the conditioned media were determined by gelatine zymography
Inhibitory effect of honey flavonoids on secreted MMP-9 in the TNF-α-stimulated HaCaT and HEK cells
Besides the inhibitory activity of HAE, we also assessed the inhibitory effect of major phenolic compounds identified in HAE on MMP-9 secretion. As shown in Fig. 3b, kaempferol and apigenin reduced the TNF-α-induced MMP-9 secretion in a dose-dependent manner without affecting the cell viability (data not shown). Ferulic and p-coumaric acids, the most abundant phenolic compounds in the HAE, did not affect the elevated levels of MMP-9. Similarly, quercetin, the potent anti-inflammatory drug, was not able to reduce MMP-9 in a dose-dependent manner.
To confirm the obtained results, we tested inhibitory effect of individual honey phenolic compounds on secreted MMP-9 in the TNF-α-stimulated HEK cells. A similar reduction of MMP-9 secretion by apigenin and kaempferol was observed in HEK cells (Fig. 3b).
Discussion
MMP-9 is a type IV collagenase that is transiently expressed in the process of normal wound healing, but it has also been found at elevated levels in chronic wounds. Prolonged and excessive production of the MMP-9 protease leads to impaired wound healing [36]. Therefore, inhibition of MMP-9 may represent a logical alternative approach in ameliorating the early stage of the impaired wound healing process.
Our data indicate that fir honeydew honey and its phenolic components have the ability to counteract destructive inflammation and re-establish normal wound healing. Pre-treatment of HaCaT cells with Sep-Pack C18 honey extract significantly reduced subsequent TNF-α-induced production of MMP-9. This pre-treatment with honey extract was accompanied with up- and down-regulation of TIMP-1 in HaCaT cells. Several potential anti-inflammatory flavonoids such as kaempferol and apigenin have been identified in HAE. This result suggests that honey-associated reduction of MMP-9 in wound fluids represents an anti-inflammatory action of honey and, thus, may explain its beneficial effect in treatment of infected and chronic wounds.
In fact, honey may have pro-inflammatory and anti-inflammatory properties presumably depending on the circumstances. In recent years, several studies have examined the role of honey in the stimulation of immune responses. Immunomodulatory effects were demonstrated in vitro by pro-inflammatory cytokine release from monocytic cell line and human peripheral monocytes after incubation with natural honeys [32]. Furthermore, Tonks et al. [33] supposed that a 5.8-kDa component isolated from manuka honey is responsible for TNF-α induction in human monocytes via Toll-like receptor 4. We showed that a natural acacia honey is able to stimulate TNF-α secretion from murine macrophages [17]. In our very recent study [18], we documented that honey activates HEK which are associated with an up-regulation of certain cytokines (TNF-α, IL-1β and TGF-β) and MMP-9. These results appear contradictory to the results of the present study. Therefore, we assume that honey can act as an immunomodulator with both pro-inflammatory and anti-inflammatory properties. We speculate that honey stimulates the production of inflammatory cytokines and MMP-9 from keratinocytes when a low level of an inflammatory/stimulatory mediator is present. On the other hand, if an environment is infected and inflammation is in progress, honey suppresses the production of inflammatory cytokines. This hypothesis is very promising and could result in new therapeutic advantages for the treatment of cutaneous inflammation in the future.
Similarly, honey and its components are able to either stimulate or inhibit the release of certain cytokines (TNF-α, IL-1β IL-6) and reactive oxygen species from human monocytes/macrophages and neutrophils, depending on the microenvironment.
On the other hand, honey also exhibits potent multiple anti-inflammatory effects. Clinically, there have been numerous observations reported of honey reducing oedema and exudate, minimising scarring and having a soothing effect when applied to inflamed wounds and burns (reviewed in [21]). The anti-inflammatory effect of honey may be explained by several mechanisms of action: (i) inhibition of the classical complement pathway [35], (ii) inhibition of reactive oxygen species formation [35], (iii) inhibition of leucocyte infiltration [14] and (iv) inhibition of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) expression [7]. Finally, the inhibition of MMP-9 production in keratinocytes reported here represents another novel anti-inflammatory mechanism of honey action.
Phenolic compounds, including flavonoids, are primarily responsible for the anti-inflammatory effect of honey [1, 7, 9, 35]. Chrysin was shown to be an effective anti-inflammatory compound [5, 37]. It suppresses LPS-induced COX-2 expression through the inhibition of nuclear factor for IL-6 DNA-binding activity [37] and inhibits the release of NO and pro-inflammatory cytokines such as TNF-α and IL-1β [5]. In the present study, we identified two flavonoids in HAE, namely apigenin and kaempferol, as suppressors of TNF-α-induced MMP-9 expression in HaCaT. Our result nicely complements a very recent study, where apigenin inhibited TNF-α-induced MMP-9 expression via modulating Akt signalling in endothelial cells [26]. In addition, apigenin effectively inhibited IL-1β-induced MMP-9 mRNA expression in osteoblasts [38]. Relatively little is known about the effects of kaempferol on MMP-9 induction and expression; however, a recent theoretical study identified kaempferol as a potent inhibitor of MMP-2 and MMP-9 activities [15].
In conclusion, our data constitute the first evidence about honey-mediated attenuation of TNF-α-induced MMP-9 expression in HaCaT and suggest that the flavonoid content of honey is able to down-regulate the expression of MMP-9, a key inflammatory mediator responsible for destructive effects in chronic wounds. Further studies are needed to determine whether honeydew honey with content of certain flavonoids would also be able to inhibit production of MMP-9 directly in wound environment.
References
Bashkaran K, Zunaina E, Bakiah S, Sulaiman SA, Sirajudeen K, Naik V (2011) Anti-inflammatory and antioxidant effects of Tualang honey in alkali injury on the eyes of rabbits: experimental animal study. BMC Complement Altern Med 11:90
Eming SA, Krieg T, Davidson JM (2007) Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 127:514–525
Fu X, Li X, Cheng B, Chen W, Sheng Z (2005) Engineered growth factors and cutaneous wound healing: success and possible questions in the past 10 years. Wound Repair Regen 13:122–130
Gill SE, Parks WC (2008) Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 40:1334–1347
Ha SK, Moon E, Kim SY (2010) Chrysin suppresses LPS-stimulated proinflammatory responses by blocking NF-κB and JNK activations in microglia cells. Neurosci Lett 485:143–147
Hirano H, Watanabe T (1990) Microsequencing of proteins electrotransferred onto immobilizing matrices from polyacrylamide gel electrophoresis: application to an insoluble protein. Electrophoresis 11:573–580
Hussein SZ, Mohd Yusoff K, Makpol S, Mohd Yusof YA (2012) Gelam honey inhibits the production of proinflammatory, mediators NO, PGE(2), TNF-α, and IL-6 in carrageenan-induced acute paw edema in rats. Evid Based Complement Alterna Med 2012 (Article ID 109636):1–12
Kassim M, Achoui M, Mansor M, Yusoff KM (2010) The inhibitory effects of Gelam honey and its extracts on nitric oxide and prostaglandin E(2) in inflammatory tissues. Fitoterapia 81:1196–1201
Kassim M, Achoui M, Mustafa MR, Mohd MA, Yusoff KM (2010) Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity. Nutr Res 30:650–659
Kleiner D, Stetler-Stevenson W (1994) Quantitative zymography: detection of pictogram quantities of gelatinases. Anal Biochem 218:325–329
Kwakman PH, te Velde AA, De Boer L, Speijer D, Vandenbroucke-Grauls CM, Zaat SA (2010) How honey kills bacteria. FASEB J 24:2576–2582
Kyriakides TR, Wulsin D, Skokos EA, Fleckman P, Pirrone A, Shipley JM, Senior RM, Bornstein P (2009) Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis. Matrix Biol 28:65–73
Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS (2002) Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen 10:26–37
Leong AG, Herst PM, Harper JL (2012) Indigenous New Zealand honeys exhibit multiple anti-inflammatory activities. Innate Immun 18:459–466
Li DL, Zheng QC, Fang XX, Ji HT, Yang JG, Zhang HX (2009) Theoretical study on potency and selectivity of novel nonpeptide inhibitors of matrix metalloproteinases mmp-2 and mmp-9. J Theor Comput Chem 8:491–506
Liu YC, Margolis DJ, Isseroff RR (2011) Does inflammation have a role in the pathogenesis of venous ulcers? A critical review of the evidence. J Invest Dermatol 131:818–827
Majtan J, Kovacova E, Bilikova K, Simuth J (2006) The immunostimulatory effect of the recombinant apalbumin 1-major honeybee royal jelly protein-on TNFα release. Int Immunopharmacol 6:269–278
Majtan J, Kumar P, Majtan T, Walls AF, Klaudiny J (2010) Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp Dermatol 19:e73–e79
Mäkelä M, Salo T, Larjava H (1998) MMP-9 from TNF alpha-stimulated keratinocytes binds to cell membranes and type I collagen: a cause for extended matrix degradation in inflammation? Biochem Biophys Res Commun 253:325–335
Mirastschijski U, Schnabel R, Claes J, Schneider W, Agren MS, Haaksma C, Tomasek JJ (2010) Matrix metalloproteinase inhibition delays wound healing and blocks the latent transforming growth factor-beta1-promoted myofibroblast formation and function. Wound Repair Regen 18:223–234
Molan PC (2011) The evidence and the rationale for the use of honey as a wound dressing. Wound Pract Res 19:204–220
Molan PC (2006) The evidence supporting the use of honey as a wound dressing. Int J Low Extrem Wounds 5:40–54
Moor AN, Vachon DJ, Gould LJ (2009) Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Repair Regen 17:832–839
Mustoe TA, Cutler NR, Allman RM, Goode PS, Deuel TF, Prause JA, Bear M, Serdar CM, Pierce GF (1994) A phase II study to evaluate recombinant platelet-derived growth factor-BB in the treatment of stage 3 and 4 pressure ulcers. Arch Surg 129:213–219
Nagase H, Woessner JF (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494
Palmieri D, Perego P, Palombo D (2012) Apigenin inhibits the TNFα-induced expression of eNOS and MMP-9 via modulating Akt signalling through oestrogen receptor engagement. Mol Cell Biochem 371:129–136
Ranzato E, Martinotti S, Burlando B (2012) Epithelial mesenchymal transition traits in honey-driven keratinocyte wound healing: comparison among different honeys. Wound Repair Regen 20:778–785
Serra R, Buffone G, Falcone D, Molinari V, Scaramuzzino M, Gallelli L, de Franciscis S (2013) Chronic venous leg ulcers are associated with high levels of metalloproteinases-9 and neutrophil gelatinase-associated lipocalin. Wound Repair Regen 21:395–401
Soo C, Shaw WW, Zhang X, Longaker MT, Howard EW, Ting K (2000) Differential expression of matrix metalloproteinases and their tissue-derived inhibitors in cutaneous wound repair. Plast Reconstr Surg 105:638–647
Steed DL (1995) Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic Ulcer Study Group. J Vasc Surg 21:71–78
Tarnuzzer RW, Schultz GS (1996) Biochemical analysis of acute and chronic wound environments. Wound Repair Regen 4:321–325
Tonks AJ, Cooper RA, Jones KP, Blair S, Parton J, Tonks A (2003) Honey stimulates inflammatory cytokine production from monocytes. Cytokine 21:242–247
Tonks AJ, Dudley E, Porter NG, Parton J, Brazier J, Smith EL, Tonks A (2007) A 5.8-kDa component of manuka honey stimulates immune cells via TLR4. J Leukoc Biol 82:1147–1155
Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G (1999) Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 7:442–452
van den Berg AJ, van den Worm E, van Ufford HC, Halkes MJ, Hoekstra MJ, Beukelman CJ (2008) An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J Wound Care 17:172–178
Widgerow AD (2012) Cellular resolution of inflammation–catabasis. Wound Repair Regen 20:2–7
Woo KJ, Jeong YJ, Inoue H, Park JW, Kwon TK (2005) Chrisin suppresses lipopolysaccharide-induced cycloooxygenase-2 expression through the inhibition of nuclear factor for IL-6 (NF-IL-6) DNA-binding activity. FEBS Lett 579:705–711
Yang H, Liu Q, Ahn JH, Kim SB, Kim YC, Sung SH, Hwang BY, Lee MK (2012) Luteolin downregulates IL-1β-induced MMP-9 and -13 expressions in osteoblasts via inhibition of ERK signalling pathway. J Enzyme Inhib Med Chem 27:261–266
Acknowledgments
This work was supported by the Slovak Research and Development Agency under the Contract No. APVV-0115-11 and partially by the Operational Program of Research and Development and co-financed with the European Fund for Regional Development (EFRD). Grant: ITMS 26240220030: Research and development of new biotherapeutic methods and its application in some illnesses treatment.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Majtan, J., Bohova, J., Garcia-Villalba, R. et al. Fir honeydew honey flavonoids inhibit TNF-α-induced MMP-9 expression in human keratinocytes: a new action of honey in wound healing. Arch Dermatol Res 305, 619–627 (2013). https://doi.org/10.1007/s00403-013-1385-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-013-1385-y