Abstract
Purpose
Canc er is the second leading cause of death in children in developed countries and most of childhood malignancies can be treated with chemo-radiotherapy. Although radiation therapy is a successful treatment modality in cancer patients, it has various adverse effects. Especially the gonads are very sensitive and prone to radiation-related damage. Radiation impairs the ovaries by triggering apoptosis of follicular cells and chromosomal damage and oxidative stress. Shilajit, a traditional medicinal agent in India, Russia, and other parts of the world, contains various antioxidant agents and has ovogenic effects. To evaluate the ability of shilajit to prevent radiation-induced ovarian damage.
Methods
Forty Wistar albino female rats were divided into four groups as: Control group, shilajit group, radiation only group, and radiation + shilajit group. Four days after radiation exposure, the rats were sacrificed and the ovaries were removed and evaluated immuno-histopathologically.
Results
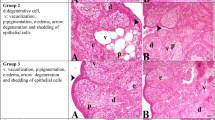
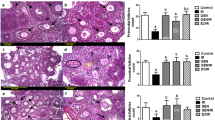
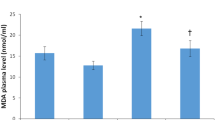
There was a statistically significant difference in follicle counts (primordial, primary, preantral, antral, and atretic follicles) between the groups (p < 0.001). Almost all follicles at all stages were atretic in the radiation only group whereas normal-looking primordial follicles were detected in the radiation + shilajit group. In radiation + shilajit group, p53, Bax and caspase 3 expression was less intense than that in the radiation only group follicles.
Conclusion
This is the first reported study evaluating the effects of shilajit on radiation-related ovarian damage prevention. Shilajit decreased the expression of p53, Bax, and caspase 3, thereby blocking the apoptotic pathways. Shilajit was found to be especially protective of primordial follicles.






Similar content being viewed by others
References
Kaatsch P (2010) Epidemiology of childhood cancer. Cancer Treat Rev 36(4):277–285
Bath LE, Hamish W, Wallace B, Critchley HOD (2002) Late effects of the treatment of childhood cancer on the female reproductive system and the potential for fertility preservation. BJOG 109(2):107–114
Miller RW, Young JL, Novakovic B (1995) Childhood cancer. Cancer 75(1):395–405
Delaney G, Jacob S, Featherstone C, Barton M (2005) The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 104(6):1129–1137
Hudson MM (2010) Reproductive outcomes for survivors of childhood cancer. Obstet Gynecol 116(5):1171–1183
Geenan M, Cardous-Ubbink M, Kremer L et al (2007) Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 297(24):2705–2715
Oeffinger KC, Mertens AC, Sklar CA et al (2006) Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355(15):1572–1582
Shinomiya N (2001) New concepts in radiation-induced apoptosis: ‘premitotic apoptosis’ and ‘postmitotic apoptosis’. J Cell Mol Med 5(3):240–253
Feinendegen LE (2002) Reactive oxygen species in cell responses to toxic agents. Hum Exp Toxicol 21(2):85–90
England K, Cotter TG (2005) Direct oxidative modifications of signalling proteins in mammalian cells and their effects on apoptosis. Redox Rep 10(5):237–245
Chemaitilly W, Mertens AC, Mitby P et al (2006) Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab 91(5):1723–1728
Pouilles JM, Tremollieres F, Bonneu M, Ribot C (1994) Infl uence of early age at menopause on vertebral bone mass. J Bone Miner Res 9(3):311–315
Hendry JH, West CM (1997) Apoptosis and mitotic cell death: their relative contributions to normal-tissue and tumour radiation response. Int J Radiat Biol 71(6):709–719
Chapman RM (1982) Effect of cytotoxic therapy on sexuality and gonadal function. Semin Oncol 9(1):84–94
Kim JK, Lee CJ, Song KW, Do BR, Yoon YD (1999) γ-Radiation accelerates follicular atresia in immautre mice. Vivo 13(1):21–24
Kaya H, Delibas N, Serteser M, Ulukaya E, Ozkaya O (1999) The effect of melatonin on lipid peroxidation during radiotherapy in female rats. Strahlenther Onkol 175(6):285–288
Abedelahi A, Salehnia M, Allameh AA (2008) The effects of different concentrations of sodium selenite on the in vitro maturation of preantral follicles in serum-free and serum supplemented media. J Assist Reprod Genet 25(9–10):483–488
Morita Y et al (2000) Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med 6(10):1109–1114
Simsek Y et al (2012) Ameliorative effects of resveratrol on acute ovarian toxicity induced by total body irradiation in young adult rats. J Pediatr Adolesc Gynecol 25(4):262–266
Rajesh K, Witt M, Anwer MK, Agarwal SP, Koch BP (2008) Spectroscopic characterization of fulvic acids extracted from the rock exudate shilajit. Org Geochem 39(12):1719–1724
Ghosal S, Lal J, Singh SK, Goel RK, Jaiswal AK, Bhattacharya SK (1991) The need for formulation of shilajit by its isolated active constituents. Phytother Res 5(5):211–216
Frolova LN, Kiseleva TL (1996) Chemical composition of mumijo and methods for determining its authenticity and quality (a review). Pharm Chem J 30(8):543–547
Al-Himaidi AR, Mohammed U (2003) Safe use of salajeet during the pregnancy of Female mice. Online J Biol Sci 3(8):681–684
Agarwal SP, Khanna R, Karmarkar R, Anwer MK, Khar RK (2007) Shilajit: a review. Phytother Res 21(5):401–405 (review)
Park Jeong-Soog, Kim Gee-Young, Han Kun (2006) The spermatogenic and ovogenic effects of chronically administered shilajit to rats. J Ethnopharmacol 107(3):349–353
Biswas TK, Pandit S, Mondal S, Biswas SK, Jana U, Ghosh T, Tripathi PC, Debnath PK, Auddy RG, Auddy B (2009) Clinical evaluation of spermatogenic activity of processed shilajit in oligospermia. Andrologia 42(1):48–56
Velmurugan C, Vivek B, Wilson E, Bharathi T, Sundaram T (2012) Evaluation of safety profile of black shilajit after 91 days repeated administration in rats. Asian Pac J Trop Biomed 2(3):210–214
Stohs SJ (2014) Safety and efficacy of shilajit (mumie, moomiyo). Phytother Res 28(4):475–479
Devine PJ, Payne CM, McCuskey MK, Hoyer PB (2000) Ultrastructural evaluation of oocytes during atresia in rat ovarian follicles. Biol Reprod 63(5):1245–1252
Qian H, Xu J, Lalioti MD, Gulle K, Sakkas D (2010) Oocyte numbers in the mouse increase after treatment with 5-aminoisoquinolinone: a potent inhibitor of poly(ADP-ribosyl)ation. Biol Reprod 82:1000–1007
Baker TG (1971) Comparative aspects of the effects of radiation during oogenesis. Mutat Res 11(1):9–22
Gosden Roger (1997) Norah spears. Programmed cell death in the reproductive system. Br Med Bull 52(3):644–661
Acknowledgments
The present study was funded by Bulent Ecevit University, Scientific Research Grant (2012-20-00-33).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Kececi, M., Akpolat, M., Gulle, K. et al. Evaluation of preventive effect of shilajit on radiation-induced apoptosis on ovaries. Arch Gynecol Obstet 293, 1255–1262 (2016). https://doi.org/10.1007/s00404-015-3924-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3924-6