Abstract
This study aims to constrain the nature of kimberlite–xenolith reactions and the fluid origin for Kimberley-type pyroclastic kimberlite (KPK). KPKs are characterized by an abundance of basement xenoliths (15–90%) and display distinct pipe morphology, textures, and mineralogy. To explain the KPK mineralogy deviating from the mineralogy of crystallized kimberlite melt, we study reactions between hypabyssal kimberlite transitional to KPK and felsic xenoliths. Here, we characterize the pectolite–diopside–phlogopite–serpentine–olivine common zonal patterns using petrography, bulk composition, thermodynamic modelling, and conserved element ratio analysis. To replicate the observed mineral assemblages, we extended the thermodynamic database to include pectolite, using calculated density functional theory methods. Our modelling reproduces the formation of the observed distinct mineralogy in reacted granitoid and gneiss. The assimilation of xenoliths is a process that starts from high temperatures (1200–600 °C) with the formation of clinopyroxene and wollastonite, continues at 600–200 °C with the growth of clinopyroxene, garnet, and phlogopite finishing at temperatures < 300 °C when pectolite and prehnite join in. Critically, the majority of the new mineral growth occurs in the sub-solidus, at temperatures below 600 °C. The metasomatic origin of the xenolith mineralogy is best explained by gradients in the chemical potentials of Si, Al, Ca, and Mg across the xenolith–kimberlite contacts. The low-temperature mineralogy of the fluid-limited thermodynamic calculations, where H2O and CO2 are controlled by kimberlite concentrations, reproduces the observed mineralogy better than a fluid-saturated model with a meteoric fluid composition. Our findings imply the deuteric origin of the fluids in KPK pipes controlling the kimberlite mineralogy and texture.









Similar content being viewed by others
Availability of data and material
Data are available as an Electronic Supplementary Material.
Code availability
Not applicable.
References
Afanasyev A, Melnik O, Poritt L, Schumacher J, Sparks RSJ (2014) Hydrothermal alteration of kimberlite by convective flows of external water. Contrib Miner Petrol 168(1):1038
Anenburg M, Mavrogenes J (2018) Carbonatitic versus hydrothermal origin for fluorapatite REE-Th deposits: experimental study of REE transport and crustal “antiskarn” metasomatism. Am J Sci 318:335–366
Atkinson WW, Einaudi MT (1978) Skarn formation and mineralization in the Contact Aureole at Carr Fork, Bingham, Utah. Econ Geol 73:1326–1365
Barton MD, Staude J-M, Snow EA, Johnson DA (1991) Aureole systematics. In: Kerrick DM (ed) Contact metamorphism. Mineralogical Society of America, Reviews in Mineralogy and Geochemistry, vol 26, pp 723–847
Benisek A, Dachs E (2018) The accuracy of standard enthalpies and entropies for phases of petrological interest derived from density-functional calculations. Contrib Miner Petrol 173(11)
Benisek A, Kroll H, Dachs E (2012) The heat capacity of fayalite at high temperatures. Am Miner 97:657–660
Birkett T, McCandless T, Hood C (2004) Petrology of the Renard igneous bodies: host rocks for diamond in the northern Otish Mountains region, Québec. Lithos 76:475–490
Buse B, Schumacher J, Sparks S, Field M (2010) Growth of bultfonteinite and hydrogarnet in metasomatized basalt xenoliths in the B/K9 kimberlite, Damtshaa, Botswana: insights into hydrothermal metamorphism in kimberlite pipes. Contrib Miner Petrol 160:533–550
Caro G, Kopylova MG, Creaser RA (2004) The hypabyssal 5034 kimberlite of the Gahcho Kuè cluster, southeastern Slave craton, Northwest Territories, Canada: a granite-contaminated Group-I kimberlite. Can Mineral 42:183–207
Cas RAF, Hayman PC, Pittari A, Porritt L (2008) Some major problems with existing models and terminology associated with kimberlite pipes from a volcanological perspective and some suggestions. J Volcanol Geoth Res 174(1):209–225
Chayes F (1962) Numerical correlation and petrographic variation. J Geol 70:440–452
Clement CR, Reid AM (1989) The origin of kimberlite pipes: an interpretation based on the synthesis of geological features displayed by southern African occurrences. In: Ross J, Jaques AL, Fergusan J, Green DH, O’Reilly SY, Danchin RV, Janse AJA (eds) Kimberlites and Related Rocks, vol 14. Geological Society of Australia, Sydney, pp 632–646
Connolly JAD (2005) Computation of phase equilibria by linear programming: a tool for geodynamic modeling and its application to subduction zone decarbonation. Earth Planet Sci Lett 236:524–541
Connolly JAD (2009) The geodynamic equation of state: what and how. Geochem Geophys Geosyst 10:Q10014
Dachs E, Benisek A (2011) A sample-saving method for heat capacity measurements on powders using relaxation calorimetry. Cryogenics 51(8):460–464
Dale J, Holland T, Powell R (2000) Hornblende-garnet-plagioclase thermobarometry: a natural assemblage calibration of the thermodynamics of hornblende. Contrib Miner Petrol 140:353–362
Dharmapriya PL, Malaviarachchi SP, Kriegsman LM, Galli A, Dyck B, Sajeev K, Pitawala A (2020) Symplectite growth in the presence of alkaline fluids: evidence from high-aluminous metasediments of the Highland Complex, Sri Lanka. Miner Petrol 114:515–538
Dyck B, Waters DJ, St-Onge MR, Searle MP (2020) Muscovite dehydration melting: Reaction mechanisms, microstructures, and implications for anatexis. J Metamorph Geol 38(1):29–52
Einaudi MT, Meinert LD, Newberry RJ (1981) Skarn deposits. In: Skinner BJ (ed) Economic geology 75th anniversary volume. The Economic Geology Publishing Company, El Paso, Texas, pp 317–391
Elliott HAL, Wall F, Chakhmouradian AR, Siegfried PR, Dahlgren S, Weatherley S, Finch AA, Marks MAW, Dowman E, Deady E (2018) Fenites associated with carbonatite complexes: a review. Ore Geol Rev 93:38–59
Evans BW (2004) The serpentine multisystem revisited: chrysotile is metastable. Int Geol Rev 46:479–506
Fedortchouk Y, Canil D (2004) Intensive variables in kimberlite magmas, Lac deGras, Canada and implications for diamond survival. J Petrol 45(9):1725–1745
Fitzgerald C, Hetman C, Lepine C, Skelton D, McCandless T (2009) The internal geology and emplacement history of the Renard 2 kimberlite, Superior Province, Québec, Canada. Lithos 112(1):513–528
Fontana G, Mac Niocaill C, Brown RJ, Sparks SJ, Field M (2011) Emplacement temperatures of pyroclastic and volcaniclastic deposits in kimberlite pipes in southern Africa. Bull Volcanol 73:1063–1083
Frantz GW, Wyllie PJ (1967) Experimental studies in the system CaO–MgO–SiO2–H2O–CO2. In: Wyllie PJ (ed) Ultramafic and related rocks. John Wiley and Sons, New York, pp 323–326
Fulop A, Kopylova M, Kurszlaukis S, Hilchie L, Ellemers P, Squibb C (2018) Petrography of the Snap Lake kimberlite dyke (Northwest Territories, Canada) and its interaction with country rock granitoids. J Petrol 59(12):2493–2518
Gaudet M, Kopylova M, Muntener C, Zhuk V, Nathwani C (2018) Geology of the Renard 65 kimberlite pipe, Québec, Canada. Mineral Petrol 112(2):433–445
Godin P, Hopkins R, Bedell P (2016) Updated Renard diamond project mine plan and mineral reserve estimate, Québec, Canada. NI 43-101 technical report
Grant JA (1986) The isocon diagram; a simple solution to Gresens’ equation for metasomatic alteration. Econ Geol 81:1976–1982
Hayman PC, Cas RAF, Johnson M (2009) Characteristics and alteration origins of matrix minerals in volcaniclastic kimberlite of the Muskox pipe (Nunavut, Canada). Lithos 112(Suppl 1):473–485
Hetman CM (2008) Tuffisitic kimberlite (TK): a Canadian perspective on a distinctive textural variety of kimberlite. J Volcanol Geoth Res 174:57–67
Hetman C, Scott Smith B, Paul J, Winter F (2004) Geology of the Gahcho Kué kimberlite pipes, NWT, Canada: root to diatreme magmatic transition zones. Lithos 76:51–74
Hilchie L, Russell J, Stanley C (2018) Unification of isocon and Pearce element ratio techniques in the quantification of material transfer. Econ Geol 113:1603–1608
Holland TJB, Powell R (1991) A compensated-Redlich-Kwong (CORK) equation for volumes and fugacities of CO2 and H2O in the range 1 bar to 50 kbar and 100–1600 °C. Contrib Miner Petrol 109:265–273
Holland T, Powell R (1996) Thermodynamics of order-disorder in minerals. 2. Symmetric formalism applied to solid solutions. Am Miner 81:1425–1437
Holland TJB, Powell R (1998) An internally consistent thermodynamic data set for phases of petrological interest. J Metamorph Geol 16:309–343
Holland T, Powell R (2001) Calculation of phase relations involving haplogranitic melts using an internally consistent thermodynamic dataset. J Petrol 42:673–683
Holland T, Powell R (2003) Activity-composition relations for phases in petrological calculations: an asymmetric multicomponent formulation. Contrib Miner Petrol 145:492–501
Holland TJB, Powell R (2011) An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. J Metamorph Geol 29:333–383
Kavanagh J, Sparks RSJ (2009) Temperature changes in ascending kimberlite magmas. Earth Planet Sci Lett 286(3–4):404–413
Kopylova MG, Matveev S, Raudsepp M (2007) Searching for parental kimberlite melt. Geochim Cosmochim Acta 71:3616–3629
Lépine I, Farrow D (2018) 3D geological modelling of the Renard 2 kimberlite pipe, Québec, Canada: from exploration to extraction. Miner Petrol 112:411–419
Lustrino M, Luciani N, Stagno V (2019) Fuzzy petrology in the origin of carbonatitic/pseudocarbonatitic Ca-rich ultrabasic magma at Polino (central Italy). Sci Rep 9: article 9212
Meinert LD, Dipple GM, Nicolescu S (2005) World skarn deposits. The economic geology 100th anniversary volume. Society of Economic Geologists Inc, Littleton, CO, pp 299–336
Mitchell RH (2008) Petrology of hypabyssal kimberlites: relevance to primary magma compositions. J Volcanol Geoth Res 174(1–3):1–8
Mitchell RH (2013) Paragenesis and oxygen isotopic studies of serpentine in kimberlite. In: Pearson D, Grutter H, Harris J, Kjarsgaard B, O’Brien H, Chalapathi Rao N, Sparks S (eds) Proceedings of the 10th international Kimberlite conference. Special Issue of the Journal of the Geological Society of India, pp. 1–12
Mitchell RH, Skinner EMW, Scott Smith BH (2009) Tuffisitic kimberlites from the Wesselton Mine, South Africa: mineralogical characteristics relevant to their formation. Lithos 112:452–464
Mitchell RH, Giuliani A, O’Brien H (2019) What is a Kimberlite? Petrology and mineralogy of hypabyssal kimberlites. Elements 15(6):381–386
Moussallam Y, Morizet Y, Massuyeau M, Laumonier M, Gaillard F (2015) CO2 solubility in kimberlite melts. Chem Geol 418:198–205
Moussallam Y, Médard E, Georgeais G, Rose-Koga EF, Koga KT, Pelletier B, Bani P, Shreve TL, Grandin R, Boichu M, Tari D, Peters N (2021) How to turn off a lava lake? A petrological investigation of the 2018 intra-caldera and submarine eruptions of Ambrym volcano. Bull Volcanol 83:36. https://doi.org/10.1007/s00445-021-01455-2
Moussallam Y, Gaillard YMF (2016) H2O–CO2 solubility in low SiO2-melts and the unique mode of kimberlite degassing and emplacement. Earth Planet Sci Lett 447:151–160
Moussallam Y, Médard E, Georgeais G, Rose-Koga EF, Koga KT, Pelletier B, Bani P, Shreve TL, Grandin R, Boichu M, Tari D, Peters N (2021) How to turn off a lava lake? A petrological investigation of the 2018 intra-caldera and submarine eruptions of Ambrym volcano. Bull Volcanol 83:36. https://doi.org/10.1007/s00445-021-01455-2
Muntener C, Gaudet M (2018) Geology of the Renard 2 pipe to 1000 m depth, Renard Mine, Québec, Canada: insights into Kimberley-type pyroclastic kimberlite emplacement. Miner Petrol 112:411–419
Muntener C, Scott Sith B (2013) Economic geology of Renard 3, Québec, Canada: A diamondiferous, multi-phase pipe, infilled with hypabyssal and tuffisitic kimberlite. In: Pearson D, Grutter H, Harris J, Kjarsgaard B, O’Brien H, Chalapathi Rao N, Sparks S (eds) Proceedings of the 10th international Kimberlite conference. Special Issue of the Journal of the Geological Society of India, vol 2, pp 241–256
Newton D, Ryan A, Hilchie L (2017) Competence and lithostratigraphy of host rocks govern kimberlite pipe morphology. Can J Earth Sci 55(2)
Nicholls J (1988) The statistics of Pearce element ratios and the Chayes closure problem. Contrib Miner Petrol 99:11–24
Niyazova S, Kopylova M, Gaudet M, De Stefano A (2021) Petrographic and geochemical characteristics associated with felsic xenolith assimilation in kimberlite. Can Mineral. https://doi.org/10.3749/canmin.2000107
Otto JW, Wyllie PJ (1993) Relationships between silicate melts and carbonate-precipitating melts in CaO–MgO–SiO2– CO2–H2O at 2 kbar. Mineral Petrol 48:343–365
Pearce TH (1968) A contribution to the theory of variation diagrams. Contrib Miner Petrol 19:142–157
Pell J, Russell JK, Zhang S (2015) Kimberlite emplacement temperatures from conodont geothermometry. Earth Planet Sci Lett 411:131–141
Percival JA, Bleeker W, Cook FA, Rivers T, Ross G, van Staal CR (2004) Panlithoprobe Workshop IV: Intra-orogen correlations and comparative orogenic anatomy. Geosci Can 31:23–39
Percival JA, Sanborn-Barrie M, Stott G, Helmstaedt H, Skulski T (2006) Tectonic evolution of the Western Superior Province from NATMAP and Lithoprobe studies. Can J Earth Sci 43:1085–1117
Pirajno F (2008) Hydrothermal processes and mineral systems. Springer Science & Business Media, Berlin, p 1250
Porritt LA, Cas RAF, Schaefer B, McKnight SW (2012) Textural analysis of strongly altered kimberlite: examples from the Ekati diamond mine, NWT, Canada. Can Miner 50(3):625–641
Powell R, Holland T (1999) Relating formulations of the thermodynamics of mineral solid solutions: Activity modeling of pyroxenes, amphiboles, and micas. Am Miner 84:1–14
Ranger I, Heaman L, Pearson D, Laroulandie C, Lépine I, Zhuk V (2017) Punctuated, long-lived emplacement history of the Renard 2 kimberlite, Canada, revealed by new high precision U-Pb groundmass perovskite dating. In: 11th International Kimberlite Conference, Botswana, 18-22 August 2017. Extended Abstracts, p 11
Russell JK, Nicholls J (1988) Analysis of petrologic hypotheses with Pearce element ratios. Contrib Miner Petrol 99:25–35
Scott Smith BH (2008) Canadian kimberlites: geological characteristics relevant to emplacement. J Volcanol Geoth Res 174:9–19
Scott Smith BH, Smith SCS (2009) The economic implications of kimberlite emplacement. In: Foley S, Aulbach S, Brey G, Grütter H, Höfer H, Jacob D, Lorenz V, Stachel T, Woodland A (eds) Proceedings of the 9th international Kimberlite conference. Lithos, vol 112, Supplement 1, pp 10–22
Scott Smith BH, Nowicki TE, Russell JK, Webb KJ, Mitchell RH, Hetman CM, Harder M, Skinner EMW, Robey JV (2013) Kimberlite terminology and classification. In: Proceedings of the 10th international kimberlite conference. Special issue of the Journal of the Geological Society of India, vol 2, pp 1–17
Scott Smith BH, Nowicki TE, Russell JK, Webb KJ, Mitchell RH, Hetman CM, Robey JV (2018) A glossary of kimberlite and related terms. Scott-Smith Petrology Inc., North Vancouver, Canada, p 259
Skinner EMW, Clement CR (1979) Mineralogical classification of southern African kimberlites. In: Proceedings of 2nd international kimberlite conference. American Geophysical Union, Washington D.C., pp 129–139
Skinner EMW, Marsh JS (2004) Distinct kimberlite pipe classes with contrasting eruption processes. In: Proceedings of the 8th international kimberlite conference. Lithos, vol 76, pp 183–200
Sparks RSJ (2013) Kimberlite volcanism. Annu Rev Earth Planet Sci 41:497–528
Sparks RSJ, Baker L, Brown R, Field M, Schumacher J, Stripp G, Walters A (2006) Dynamical constraints on kimberlite volcanism. J Volcanol Geoth Res 155:18–48
Sparks RSJ, Brooker R, Field M, Kavanagh J, Schumacher J, Walters M, White J (2009) The nature of erupting kimberlite melts. Lithos 112S:429–438
Stasiuk LD, Lockhart GD, Nassichuk WW (1999) Thermal maturity evaluation of dispersed organic matter inclusions from kimberlite pipes, Lac de Gras, Northwest Territories, Canada. Int J Coal Geol 40:1–25
Stripp GR, Field M, Schumacher JC, Sparks RSJ, Cressey G (2006) Post-emplacement serpentinization and related hydrothermal metamorphism in a kimberlite from Venetia, South Africa. J Metamorph Geol 24:515–534
Van Straaten BI, Kopylova MG, Russell JK, Scott Smith BH (2011) A rare occurrence of crater-filling clastogenic extrusive coherent kimberlite, Victor Northwest (Ontario, Canada). Bull Volcanol 73(8):1047–1062
White RW, Powell R, Holland TJB (2001) Calculation of partial melting equilibria in the system Na2O-CaO-K2O-FeO-MgO-Al2O3-SiO2-H2O (NCKFMASH). J Metamorph Geol 19:139–153
Zhang Z, Fedortchouk Y, Hanley J (2015) Evolution of diamond resorption in a silicic aqueous fluid at 1–3 GPa: application to kimberlite emplacement and mantle metasomatism. Lithos 227:179–193
Acknowledgements
We are grateful to Barbara Scott Smith and Kelly Russell for insightful discussions and support of this research project. Barbara Scott Smith is thanked for explaining the geology and textures of the Renard 65 pipe and for liaising with Stornoway Diamond Corporation. We thank Stornoway Diamond Corporation for providing sample info, bulk rock geochemical data and for permission to publish.
Funding
This research was supported by a Natural Sciences and Engineering Research Council Discovery grant to MGK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests and no competing interests.
Additional information
Communicated by Dante Canil.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix 1: Determination of thermodynamic parameters for pectolite
Thermodynamic parameters for pectolite (NaCa2Si3O8(OH)) are summarized in Table 3. The values for ∆fH298.15 and V298.15 were calculated according to Benisek and Dachs (2018) using DFT methods. CP was experimentally measured and was used to calculate the standard entropy S298.15 as described below. Thermal expansion α and bulk modulus K were estimated as mean values from diopside and jadeite. The values were manually added to the thermodynamic database file hp62ver.dat (Holland and Powell 2011).
Calorimetric methods
Low-temperature heat capacities (CP) were measured using a commercially designed relaxation calorimeter (Physical Properties Measurements System (PPMS); Quantum Design®) at temperatures between 2 and 300 K using a measuring technique described by Dachs and Benisek (2011). The sample powder (~ 10 mg) was put into an Al cup (~ 8 mg) made from an Al-foil. It was pressed to a cylindrical pellet (0.5 mm thickness, 5 mm in diameter), the Al-foil surrounding the sample powder. The heat capacity at higher temperatures was measured with a differential scanning calorimeter from Perkin Elmer (Diamond DSC®) according to the method described in Benisek et al. (2012). The measurements were performed at temperatures between 280 and 670 K on two pectolite samples from the Renard 65 pipe weighing 44 mg with compositions 52.65 wt% SiO2, 0.06 wt% Al2O3, 0.26 wt% FeO, 0.17 wt% MnO, 0.31 wt% MgO, 32.47 wt% CaO, and 8.58 wt% Na2O.
Evaluation of calorimetric data
The PPMS CP data were used to calculate the standard entropy by solving the integral of CP/T dT numerically from 0 to 298.15 K using a spline interpolation function of Mathematica®. The resulting standard entropy usually agrees with published reference values within 0.21% for silicates (Dachs and Benisek 2011). The DSC CP data were fitted to a polynomial of the following form:
The a, b, c, and d coefficients in the CP polynomial correspond to c1, c3, c5, and c2, respectively, in the hp62ver.dat file.
Appendix 2: Thermodynamic phase equilibria models
Phase equilibria models for system with components SiO2, TiO2, Al2O3, Cr2O3, FeO, MnO, MgO, CaO, Na2O, K2O, CO2, H2O) for four whole rock compositions were produced. The four bulk compositions represented fresh and moderately reacted xenoliths of both types, granitoid and gneiss (Table 4). The analyses are the subset of the larger bulk rock major element analyses database fully described in Appendix 4. Since P2O5 is not a modelled component by default in Perple_X, it is stoichiometrically deducted from the bulk chemistry in the form of apatite along with the corresponding CaO amount (3.333 CaO·P2O5, Table 4) using a procedure described in Dyck et al. (2020). The phase equilibria models were computed for analyzed contents of CO2 and H2O for fresh and moderately reacted xenoliths (Table 5) and additionally for fluid-saturated conditions for moderately reacted xenoliths (Table 6). The modelling output is represented as the modal composition of mineral phases at surface conditions in resulting Figs. 6 and 7. All phases below 5% or with very limited temperature stability intervals not greater than 10% are combined into “Other”.
Analyses (2), (4), (6), and (8) used for modelling were recalculated compositions that excluded apatite, i.e., P2O5 were equated to zero and CaO was reduced stoichiometrically.
Appendix 3: Mineralogical zonation models in chemical potential space
A generic chemical potential diagram in μ(SiO2 + Al2O3) − μMgO space is a useful tool for explaining the formation of the disequilibrium mineralogical zonal patterns observed in the completely reacted xenoliths (Fig. 5a, c). To account for the mineral phases observed, we used the simplified MgO–SiO2–Al2O3–CaO–Na2O–K2O–H2O–CO2 model system. The mineral phases involved in the reactions are quartz, plagioclase, pectolite, dioside, phlogopite, serpentine, and forsterite. Biotite and spinel are ignored. Graphical depiction of the mineral assemblage is done on a three-component compatibility diagram, illustrating positions of various stable minerals based on molar quantities of the components. We grouped oxides into MgO − (SiO2 + Al2O3) − (CaO + Na2O + K2O) to make three components, and all the reactions occur in the presence of a fluid composed of a mixture of H2O and CO2. For simplification, plagioclase is excluded from the reacting assemblage. However, since pectolite is formed by reacting plagioclase, rather than quartz, with calcite, an alternative reaction producing pectolite would be a reaction between quartz and calcite in the presence of plagioclase. To retain the importance of plagioclase as a reacting phase, it is shown as a hollow dot on the compatibility diagram.
The compatibility diagram with all the involved phases mapped (Fig. 8a) is the basis for the μ(SiO2 + Al2O3) − μMgO chemical potential diagram on which the topological relationships between the stable phases have to be preserved (Fig. 8b). To properly depict the slopes of the univariant reaction lines, stochiometric reactions between the phases, containing SiO2, Al2O3, and MgO, and excess fluids and plagioclase must be balanced. The ratio of molar proportions of MgO to (SiO2 + Al2O3) is the slope of a particular reaction.
Appendix 4: Bulk compositional profiles and conserved element ratio analysis
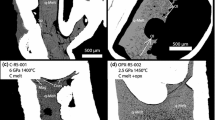
Two types of xenolith-kimberlite traverses were selected for analytical work, (i) granitoid xenoliths ranging from fresh/unaltered to those with a high degree of reaction and passing into the kimberlite reaction halo and further into hypabyssal kimberlite; and (ii) gneiss xenoliths ranging from fresh/unaltered to those with a high degree of reaction and passing into the kimberlite reaction halo and further into hypabyssal kimberlite. The samples included: for (i) CRGR1 and CRGR2—granitoid country rocks from drillcores, GRx2 and GRx3—slightly altered and moderately altered, respectively, granitoid xenoliths from drillcores, GRx1-c—moderately reacted core of a granitoid xenolith 5 mm from the contact, GRx1-r1 and GRx1-r2—highly reacted rims of a granitoid xenolith bordering kimberlite, HKh-65b—kimberlite reaction halo adjacent to granitoid xenolith, HK-65b1 to HK-65b3 and HK-65c—hypabyssal kimberlite; for (ii) CRGN1 and CRGN2—gneiss country rocks from drillcores, GNx2 and GNx3—a slightly altered and moderately altered, respectively, gneiss xenoliths from drillcores, GNx1-c—moderately reacted core of a gneiss xenolith 5 mm from the contact, GNx1-r—highly reacted rim of a gneiss xenolith bordering kimberlite, HKh-65b—kimberlite reaction halo adjacent to gneiss xenolith, HK-65b1 to HK-65b3, and HK-65c—hypabyssal kimberlite.
Bulk rock major elements were analysed in six samples (CRGR2, GRx2, GRx3, CRGN2, GNx2, GNx3) at Activation Laboratories, Ontario, Canada. Major oxides were determined by X-ray fluorescence (XRF) spectroscopy, FeO by titration, CO2 content by CO2-infrared, and structural and adsorbed water (H2O+ and H2O−) by infrared and gravimetry. CO2 and H2O are combined as a loss on ignition (LOI). FeO was determined through titration, using a cold acid digestion of ammonium metavanadate, and hydrofluoric acid in an open system. Ferrous ammonium sulphate is added after digestion and potassium dichromate is the titrating agent. H2O-(moisture) was determined gravimetrically using a 2 g sample heated in an oven at 105 °C. For analysis of H2O+, 0.3 g of the dried sample is thermally decomposed in a resistance furnace in a pure nitrogen environment at 1000 °C, using an ELTRA CW-800, directly releasing H2O+. For infrared analysis of CO2, 0.2 g sample is thermally decomposed in a resistance furnace in a pure nitrogen environment at 1000 °C, using an ELTRA instrument, directly releasing CO2 after H2O had been removed in a moisture trap. The concentration of CO2 is detected as a reduction in the level of energy at the detector. The bulk rock data for samples CRGR1, CRGN1, HK-65b2, HK-65b3 and HK-65c were provided by Stornoway Diamond Corporation. Information on drill hole and depth, locations, the host phase of kimberlite for all analyzed samples can be found in Niyazova et al. (2021).
Major element compositions for five samples from the granitoid suite (GRx1-c, GRx1-r1, GRx1-r2, HKh-65b, HK-65b1) and four samples from the gneiss suite (GNx1-c, GNx1-r, HKh-65b, HK-65b1) were analysed in polished thin sections using specially designed electron microprobe techniques. The cores and rims of the two zonally altered granitoid and gneiss xenoliths (GRx1 and GNx1) and their adjacent host kimberlite (Kimb65b) were analysed using a sequence of 2 by 2 mm2 square “frames”. Within each frame, sixty-four individual raster analyses were obtained on an 8 × 8 square grid and averaged to mimic a bulk rock analysis of a much-restricted area with the xenolith-kimberlite reaction products. Each frame was positioned over the mineralogy representative of a moderate and a high degree of reaction in the xenolith core (-c) and rims (-r), respectively, the adjacent hypabyssal kimberlite reaction halo surrounding the xenolith (HKh), and the main host (HK). For each of the nine frames major element oxide analyses were obtained on a fully automated CAMECA SX50 electron microprobe (EMP; Earth and Ocean Sciences Department, University of British Columbia), operating in a wavelength-dispersion mode with the following operating conditions: excitation voltage 15 kV, beam current 20 nA, peak count time 20 s, background count time 10 s, spot diameter 30 μm. Na and P were measured first. The resulting EPMA major oxide values are highly dependent upon the positioning of the points within the frame and the localisation of the frame upon an area capturing the representative mineralogy. To check the effect of the points positioning and the sensitivity of the analysis, we compared the averaged values of all the 64 points with two subsets of the points in the same frames. We averaged separately a subset of the odd-numbered and another subset of even-numbered points. The resulting relative difference between the 64-point averages and the subsets averages turned out to be not greater than 3% for SiO2, 14% for MgO, and 16% for CaO. The isocon analyses were done on the geochemical profiles that are created using both the bulk rock data and the unconventional EPMA data of the averaged 64 raster points. To check whether the low precision of these EPMA analyses distorts the shapes of the geochemical profiles, we created the same profiles based only on the bulk rock compositions and compared them with the original “all-inclusive” profiles, i.e., the profiles that include EPMA samples. The shapes of the bulk-composition-only profiles did not change principally; therefore, we concluded that the low accuracy of the EPMA did not distort the geochemical interpretation. Our treatment and discussion of the geochemical data, including the isocon analyses, therefore, are based on the all-inclusive geochemical profiles. The analyses for all samples are provided in the Electronic Supplementary Material.
Conserved element ratio analysis was employed to counteract the closure effect in constraining chemical processes during xenoliths’ assimilation. To aid visualization of mass transfer, we used a translation procedure of Hilchie et al. (2018) applied to an isocon analysis (Grant 1986). In the procedure, a normalized reference composition is deducted from the normalized composition of the altered samples, thus demonstrating deviations from the original composition. Samples plotting above the zero-base line represent gains of material with respect to the reference sample, and those plotting below represent losses. The data and worked procedure of the translated isocon analysis are provided in the Electronic Supplementary Material.
The conserved element ratio analysis comprised several consecutive steps:
-
(1)
Selection of the fresh reference samples, representing the original composition prior to xenolith-kimberlite reactions. Fresh unreacted granitoid and gneiss country rock samples are the original reference for the respective xenoliths, and fresh hypabyssal kimberlite away from the xenoliths is the original reference composition for the host kimberlite.
-
(2)
Selection of the conserved elements. An ideal conserved element shows none or the least variation in the suite, i.e., its amount does not change along with reactions. In the granitoid-kimberlite zonation samples, phosphorus (P) varies the least in the granitoid, and Mn varies the least in the kimberlite. Therefore, P is chosen as the conserved element for the granitoid samples and Mn for the kimberlite samples. Mn content in country rock granites is below 0.01 wt%, too low for a numerator and too close for a minimum detection limit. In the gneiss-kimberlite suite, Mn shows the least variation at higher concentrations and is chosen as the conserved element for both lithologies.
-
(3)
Conversion to molar proportions of each element per 100 g of the material:
$$\mathrm{mol\, per }\,100\mathrm{ g}\,=\,\frac{\mathrm{Oxide\, concentration }}{\mathrm{Molar\,mass\, of\, oxide}} \,\times\, \#\,\mathrm{ of \,cations\, per\, oxide\, formula}$$ -
(4)
Calculation of the molar Pearce Element Ratios (PER) by:
$$\mathrm{PER}\,=\,\frac{\mathrm{mol\,per\,}100\mathrm{\,g\,of\, Element }}{\mathrm{mol\,per\,}100\mathrm{\,g\,of\, Conserved \,Element}}$$ -
(5)
Calculation of the Translated Conserved Element Ratios by subtracting the PER of the reference samples from each sample (Tables Isocons in ESM). The reference samples plot at zero while the altered samples fluctuate around the zero-base line. Note that the observed threefold maximal variations in P in granitoids (from 0.05 to 0.16 wt% P2O5) creates a large difference in the normalized SiO2 (−1200/P) between CRGR1 and CRGR2. The change is only moderately lower than the magnitude of change in reacted granite xenoliths (samples GRx1-c, GRx1-r1, GRx1-r2) attributed to the outflow of Si.
Rights and permissions
About this article
Cite this article
Niyazova, S., Kopylova, M., Dyck, B. et al. The assimilation of felsic xenoliths in kimberlites: insights into temperature and volatiles during kimberlite emplacement. Contrib Mineral Petrol 176, 84 (2021). https://doi.org/10.1007/s00410-021-01837-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-021-01837-x