Abstract
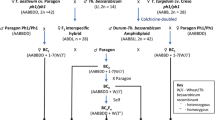
Thinopyrum bessarabicum (2n = 2x = 14, JJ or EbEb) is a valuable source of genes for bread wheat (2n = 6x = 42) improvement because of its salinity tolerance and disease resistance. Development of wheat-Th. bessarabicum translocation lines by backcrossing the amphiploid in the absence of the Ph1 gene (allowing intergenomic recombination) can assist its utilization in wheat improvement. In this study, six novel wheat-Th. bessarabicum translocation lines involving different chromosome segments (T4BS.4BL-4JL, T6BS.6BL-6JL, T5AS.5AL-5JL, T5DL.5DS-5JS, T2BS.2BL-2JL, and the whole arm translocation T1JS.1AL) were identified and characterized using genomic in situ hybridization (GISH) and fluorescent in situ hybridization (FISH). No background translocations between wheat genomes were observed. The involvement of five of the seven chromosomes and small terminal segments of Th. bessarabicum chromosome arm were important, contributing to both reduced linkage drag of the derived lines by minimizing agronomically deleterious genes from the alien species and high stability including transmission of the alien segment. All three wheat genomes were involved in the translocations with the alien chromosome, and GISH showed the Th. bessarabicum genome was more closely related to the D genome in wheat. All the introgression lines were disomic, stable, and with good morphological characters.



Similar content being viewed by others
References
Able JA, Langridge P (2006) Wild sex in the grasses. Trends Plant Sci 11:261–263. doi:10.1016/j.tplants.2006.04.004
Ali N (2012) Molecular markers, cytogenetics and epigenetics to characterize wheat-Thinopyrum hybrid lines conferring wheat streak mosaic virus resistance. Dissertation, University of Leicester
An D, Zheng Q, Zhou Y, Ma P, Lv Z, Li L, Li B, Luo Q, Xu H, Xu Y (2013) Molecular cytogenetic characterization of a new wheat–rye 4R chromosome translocation line resistant to powdery mildew. Chromosome Res 21:419–432. doi:10.1007/s10577-013-9366-8
Anamthawat-Jonsson K, Heslop-Harrison J (1993) Isolation and characterization of genome-specific DNA sequences in Triticeae species. Mol Gen Genet 240:151–158. doi:10.1007/BF00277052
Ayala-Navarrete L, Bariana H, Singh R, Gibson J, Mechanicos A, Larkin PJ (2007) Trigenomic chromosomes by recombination of Thinopyrum intermedium and Th. ponticum translocations in wheat. Theor Appl Genet 116:63–75. doi:10.1007/s00122-007-0647-5
Ayala-Navarrete L, Mechanicos A, Gibson J, Singh D, Bariana H, Fletcher J, Shorter S, Larkin PJ (2013) The pontin series of recombinant alien translocations in bread wheat: single translocations integrating combinations of Bdv2, Lr19 and Sr25 disease-resistance genes from Thinopyrum intermedium and Th. ponticum. Theor Appl Genet 126:2467–2475. doi:10.1007/s00122-013-2147-0
Bardsley D, Cuadrado A, Jack P, Harrison G, Castilho A, Heslop-Harrison J (1999) Chromosome markers in the tetraploid wheat Aegilops ventricosa analysed by in situ hybridization. Theor Appl Genet 99:300–304. doi:10.1007/s001220051236
Bedbrook J, Jones J, O’Dell M, Thompson R, Flavell R (1980) A molecular description of telomeric heterochromatin in Secale species. Cell 19:545–560. doi:10.1016/0092-8674(80)90529-2
Borlaug NE (1983) Contributions of conventional plant breeding to food production. Science 219:689–693. doi:10.1126/science.219.4585.689
Brown JW, Kemble RJ, Law CN, Flavell RB (1979) Control of endosperm proteins in Triticum aestivum (var. Chinese spring) and Aegilops umbellulata by homoeologous group 1 chromosomes. Genetics 93:189–200. doi:10.1007/s001220050413
Castilho A, Miller T, Heslop-Harrison J (1996) Physical mapping of translocation breakpoints in a set of wheat-Aegilops umbellulata recombinant lines using in situ hybridization. Theor Appl Genet 93:816–825. doi:10.1007/BF00224081
Castilho A, Miller T, Heslop-Harrison J (1997) Analysis of a set of homoeologous group 1 wheat-Aegilops umbellulata recombinant chromosome lines using genetic markers. Theor Appl Genet 94:293–297. doi:10.1007/s001220050413
Chen Q (2005) Detection of alien chromatin introgression from Thinopyrum into wheat using S genomic DNA as a probe—a landmark approach for Thinopyrum genome research. Cytogenet Genome Res 109:350–359. doi:10.1159/000082419
Comai L (2000) Genetic and epigenetic interactions in allopolyploid plants. Plant Mol Biol 48:387–399. doi:10.1023/A:1006480722854
Cuadrado A, Cardoso M, Jouve N (2008) Physical organisation of simple sequence repeats (SSRs) in Triticeae: structural, functional and evolutionary implications. Cytogenet Genome Res 120:210–219. doi:10.1159/000121069
Cuadrado A, Jouve N (2002) Evolutionary trends of different repetitive DNA sequences during speciation in the genus Secale. J Hered 93:339–345. doi:10.1093/jhered/93.5.339
Danilova TV, Friebe B, Gill BS (2012) Single-copy gene fluorescence in situ hybridization and genome analysis: Acc-2 loci mark evolutionary chromosomal rearrangements in wheat. Chromosoma 121:597–611. doi:10.1007/s00412-012-0384-7
Falke K, Sušić Z, Wilde P, Wortmann H, Möhring J, Piepho H-P, Geiger H, Miedaner T (2009) Testcross performance of rye introgression lines developed by marker-assisted backcrossing using an Iranian accession as donor. Theor Appl Genet 118:1225–1238. doi:10.1007/s00122-009-0976-7
Fedak G, Han F (2005) Characterization of derivatives from wheat-Thinopyrum wide crosses. Cytogenet Genome Res 109:360–367. doi:10.1159/000082420
Feuillet C, Langridge P, Waugh R (2008) Cereal breeding takes a walk on the wild side. Trends Genet 24:24–32. doi:10.1016/j.tig.2007.11.001
Friebe B, Jiang J, Gill BS, Dyck PL (1993) Radiation-induced nonhomologous wheat-Agropyron intermedium chromosomal translocations conferring resistance to leaf rust. Theor Appl Genet 86:141–149. doi:10.1007/BF00222072
Friebe B, Jiang J, Raupp W, McIntosh R, Gill B (1996) Characterization of wheat-alien translocations conferring resistance to diseases and pests: current status. Euphytica 91:59–87. doi:10.1007/BF00035277
Friebe B, Zeller FJ, Mukai Y, Forster BP, Bartos P, Mcintosh RA (1992a) Characterization of rust-resistant wheat Agropyron intermedium derivatives by C-Banding, in situ hybridization and isozyme analysis. Theor Appl Genet 83:775–782. doi:10.1007/BF00226697
Friebe B, Mukai Y, Gill BS, Cauderon Y (1992b) C-Banding and in situ hybridization analyses of Agropyron intermedium, a partial wheat X Ag. intermedium amphiploid, and 6 derived chromosome addition lines. Theor Appl Genet 84:899–905. doi:10.1007/BF00227402
Fu S, Lv Z, Qi B, Guo X, Li J, Liu B, Han F (2012) Molecular cytogenetic characterization of wheat—Thinopyrum elongatum addition, substitution and translocation lines with a novel source of resistance to wheat Fusarium head blight. J Genet Genomics 39:103–110. doi:10.1016/j.jgg.2011.11.008
Gale M, Sharp P, Chao S, Law C (1989) Applications of genetic markers in cytogenetic manipulation of the wheat genomes. Genome 31:137–142. doi:10.1139/g89-025
Gerlach W, Dyer T (1980) Sequence organization of the repeating units in the nucleus of wheat which contain 5s rRNA genes. Nucleic Acids Res 8:4851–4865. doi:10.1093/nar/8.21.4851
Gill BS, Friebe BR, White FF (2011) Alien introgressions represent a rich source of genes for crop improvement. Proc Natl Acad Sci 108:7657–7658. doi:10.1073/pnas.1104845108
Gorham J, McDonnell E, Budrewicz E, Jones RW (1985) Salt tolerance in the Triticeae: growth and solute accumulation in leaves of Thinopyrum bessarabicum. J Exp Bot 36:1021–1031. doi:10.1093/jxb/36.7.1021
Graybosch RA, Peterson C, Baenziger PS, Baltensperger DD, Nelson LA, Jin Y, Kolmer J, Seabourn B, French R, Hein G (2009) Registration of ‘mace’ hard red winter wheat. J Plant Regist 3:51–56. doi:10.3198/jpr2008.06.0345crc
Heslop-Harrison JS (1991) The molecular cytogenetics of plants. J Cell Sci 100:15–21
Heslop-Harrison J, Leitch A, Schwarzacher T, Anamthawat-Jonsson K (1990) Detection and characterization of 1B/1R translocations in hexaploid wheat. Heredity 65:385–392. doi:10.1038/hdy.1990.108
Heslop-Harrison J, Schwarzacher T (2012) Genetics and genomics of crop domestication. In: Altman A and Hasegawa PM (ed) Plant biotechnology and agriculture: prospects for the 21st century. Elsevier, pp1-16
Hu L-J, Liu C, Zeng Z-X, Li G-R, Song X-J, Yang Z-J (2012) Genomic rearrangement between wheat and Thinopyrum elongatum revealed by mapped functional molecular markers. Genes Genom 34:67–75. doi:10.1007/s13258-011-0153-7
Islam-Faridi MN (1988) Genetical studies of grain protein and developmental charcters in wheat. Dissertation, University of Cambridge
Jiang J, Friebe B, Gill BS (1993) Recent advances in alien gene transfer in wheat. Euphytica 73:199–212. doi:10.1007/BF00036700
King I, Purdie K, Rezanoor H, Koebner R, Miller T, Reader S, Nicholson P (1993) Characterization of Thinopyrum bessarabicum chromosome segments in wheat using random amplified polymorphic DNAs (RAPIDs) and genomic in situ hybridization. Theor Appl Genet 86:895–900. doi:10.1007/BF00211038
King IP, Forster BP, Law CC, Cant KA, Orford SE, Gorham J, Reader S, Miller TE (1997) Introgression of salt-tolerance genes from Thinopyrum bessarabicum into wheat. New Phyto 137:75–81. doi:10.1046/j.1469-8137.1997.00828.x
Kishii M, Nagaki K, Tsujimoto H (2001) A tandem repetitive sequence located in the centromeric region of common wheat (Triticum aestivum) chromosomes. Chromosome Res 9:417–428. doi:10.1023/A:1016739719421
Kishii M, Dou Q, Garg M, Ito M, Tanaka H, Tsujimoto H (2010) Production of wheat-Psathyrostachys huashanica chromosome addition lines. Genes Genet Syst 85:281–286. doi:10.1266/ggs.85.281
Knott D (1961) The inheritance of rust resistance. Vi. The transfer of stem rust resistance from Agropyron elongatum to common wheat. Can J Plant Sci 41:109–123. doi:10.4141/cjps61-014
Knott D (1968) Translocations involving Triticum chromosomes and Agropyron chromosomes carrying rust resistance. Can J Genet Cytol. doi:10:695-696
Kubaláková M, Kovářová P, Suchánková P, Číhalíková J, Bartoš J, Lucretti S, Watanabe N, Kianian SF, Doležel J (2005) Chromosome sorting in tetraploid wheat and its potential for genome analysis. Genetics 170:823–829. doi:10.1534/genetics.104.039180
Kuraparthy V, Chhuneja P, Dhaliwal HS, Kaur S, Bowden RL, Gill BS (2007) Characterization and mapping of cryptic alien introgression from Aegilops geniculata with new leaf rust and stripe rust resistance genes Lr57 and Yr40 in wheat. Theor Appl Genet 114:1379–1389. doi:10.1007/s00122-007-0524-2
Liang G, Wang R, Niblett C, Heyne E (1979) Registration of B-6-37-1 wheat germplasm 1 (reg. no. GP 118). Crop Sci 19:421–421
Liu WX, Danilova TV, Rouse MN, Bowden RL, Friebe B, Gill BS, Pumphrey MO (2013) Development and characterization of a compensating wheat-Thinopyrum intermedium Robertsonian translocation with Sr44 resistance to stem rust (Ug99). Theor Appl Genet 126:1167–1177. doi:10.1007/s00122-013-2044-6
Lukaszewski AJ (1990) Frequency of 1RS.1AL and 1RS.1BL translocations in united states wheats. Crop Sci 30:1151–1153. doi:10.2135/cropsci1990.0011183X003000050041x
McIntyre C, Pereira S, Moran L, Appels R (1990) New Secale cereale (rye) DNA derivatives for the detection of rye chromosome segments in wheat. Genome 33:635–640. doi:10.1139/g90-094
Molnár-Láng M, Linc G, Friebe B, Bucsi J, Linc G, Sukta J (2012) Detection of wheat-barley translocations by genomic in situ hybridization in derivatives of hybrids multiplied in vitro. Euphytica 112:117–123. doi:10.1023/A:1003840200744
Molnár-Láng M, Linc G, Friebe BR, Sutka J (2000) Detection of wheat-barley translocations by genomic in situ hybridization in derivatives of hybrids multiplied in vitro. Euphytica 112:117–123. doi:10.1023/A:1003840200744
Mutti JS, Baenziger PS, Graybosch RA, French R, Gill KS (2011) Registration of seven winter wheat germplasm lines carrying the Wsm1 gene for wheat streak mosaic virus resistance. J Plant Reg 5:414–417. doi:10.3198/jpr2010.03.0169crg
Mujeeb-Kazi A et al (2013) Genetic diversity for wheat improvement as a conduit to food security. Adv Agron 122:179–257
Nagaki K, Tsujimoto H, Sasakuma T (1998) Dynamics of tandem repetitive Afa-family sequences in Triticeae, wheat-related species. J Mol Evol 47:183–189. doi:10.1007/PL00006375
Pedersen C, Langridge P (1997) Identification of the entire chromosome complement of bread wheat by two-colour FISH. Genome 40:589–593. doi:10.1139/g97-077
Qi Z, Du P, Qian B, Zhuang L, Chen H, Chen T, Shen J, Guo J, Feng Y, Pei Z (2010) Characterization of a wheat-Thinopyrum bessarabicum (T2JS-2BS.2BL) translocation line. Theor Appl Genet 121:589–597. doi:10.1007/s00122-010-1332-7
Rayburn AL, Gill B (1986) Molecular identification of the D-genome chromosomes of wheat. J Hered 77:253–255
Ribeiro-Carvalho C, Guedes-Pinto H, Harrison G, Heslop-Harrison JS (1997) Wheat-rye chromosome translocations involving small terminal and intercalary rye chromosome segments in the Portuguese wheat landrace Barbela. Heredity 78:539–546. doi:10.1038/hdy.1997.84
Riley R, Chapman V, Johnson R (1968a) Introduction of yellow rust resistance of Aegilops comosa into wheat by genetically induced homoeologous recombination. Nature 217:383–384. doi:10.1038/217383a0
Riley R, Chapman V, Johnson R (1968b) The incorporation of alien disease resistance in wheat by genetic interference with the regulation of meiotic chromosome synapsis. Genet Res 12:199–219. doi:10.1017/S0016672300011800
Schlegel R, Kynast R, Schwarzacher T, Römheld V, Walter A (1993) Mapping of genes for copper efficiency in rye and the relationship between copper and iron efficiency. Plant Soil 154:61–65
Schwarzacher T, Anamthawat-Jonsson K, Harrison G, Islam A, Jia J, King I, Leitch A, Miller T, Reader S, Rogers W (1992) Genomic in situ hybridization to identify alien chromosomes and chromosome segments in wheat. Theor Appl Genet 84:778–786
Schwarzacher T, Heslop-Harrison JS (2000) Practical in situ hybridization. BIOS Scientific, Oxford
Sears E (1956) The transfer of leaf-rust resistance from Aegilops umbellulata to wheat: genetics in plant breeding. Brookhaven Symposia in Biology, pp 1-22
Sears ER (1973) Agropyron-wheat transfers induced by homoeologous pairing. Proceedings, Fourth International Wheat Genetics Symposium, Columbia, MO, Agriculture Experiment Station, College of Agriculture, University of Missouri, Columbia, MO, pp 191-199
Sears ER (1977) Analysis of wheat-Agropyron recombinant chromosomes: interspecific hybridization in plant breeding. Proceedings of the 8th EUCARPIA Congress, Madrid, Spain, pp 63-72
Sepsi A, Molnár I, Szalay D, Molnár-Láng M (2008) Characterization of a leaf rust-resistant wheat–Thinopyrum ponticum partial amphiploid BE-1, using sequential multicolor GISH and FISH. Theor Appl Genet 116:825–834. doi:10.1007/s00122-008-0716-4
Sharma D, Knott D (1966) The transfer of leaf-rust resistance from Agropyron to Triticum by irradiation. Can J Genet Cytol 8:137–143. doi:10.1139/g66-018
Simmonds N (1993) Introgression and incorporation. Strategies for the use of crop genetic resources. Biol Rev 68:539–562. doi:10.1111/j.1469-185X.1993.tb01243.x
Vershinin A, Svitashev S, Gummesson P-O, Salomon B, Von Bothmer R, Bryngelsson T (1994) Characterization of a family of tandemly repeated DNA sequences in Triticeae. Theor Appl Genet 89:217–225. doi:10.1007/BF00225145
Vrána J, Kubaláková M, Simková H, Číhalíkovái J, Lysák MA, Dolezel J (2000) Flow sorting of mitotic chromosomes in common wheat (Triticum aestivum L.). Genetics 156:2033–2041
Wang R-C, Larson S, Horton W, Chatterton N (2003a) Registration of W4909 and W4910 bread wheat germplasm lines with high salinity tolerance. Crop Sci 43:746–746
Wang R-C, Li X-M, Hu Z-M, Zhang J-Y, Larson SR, Zhang X-Y, Grieve CM, Shannon MC (2003b) Development of salinity-tolerant wheat recombinant lines from wheat disomic addition line carrying a Thinopyrum junceum chromosome. Int J Plant Sci 164:25–33
Wang RRC (2011) Chapter 2. Agropyron and Psathyrostachys. In: Kole C (ed) Wild crop relatives: genomic and breeding resources: cereals. Springer, Berlin, pp 77–108. doi:10.1007/978-3-642-14228-4_2
William M, Mujeeb-Kazi A (1993) Thinopyrum bessarabicum: biochemical and cytological markers for the detection of genetic introgression in its hybrid derivatives with Triticum aestivum L. Theor Appl Genet 86:365–370
Acknowledgments
We would like to thank “Monsanto Beachell-Borlaug International Fellowship” (MBBISP) program for funding the PhD work of Chetan Patokar and the EU Marie Curie fellowship (FP7-PEOPLE-2013-IEF 625835) to Adél Sepsi. The IAEA-FAO Cooperative Research Project “Climate Proofing of Food Crops: Genetic Improvement for Adaptation to High Temperatures in Drought Prone Areas and Beyond,” D2.30.29, is acknowledged by Trude Schwarzacher and J.S. Heslop-Harrison.
Conflict of interest
The authors declare that they have no competing interests.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patokar, C., Sepsi, A., Schwarzacher, T. et al. Molecular cytogenetic characterization of novel wheat-Thinopyrum bessarabicum recombinant lines carrying intercalary translocations. Chromosoma 125, 163–172 (2016). https://doi.org/10.1007/s00412-015-0537-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-015-0537-6