Abstract
Background
Microbiome feedbacks are proposed to influence Parkinson’s disease (PD) pathophysiology. A number of studies have evaluated the impact of oral medication on the gut microbiome (GM) in PD. However, the influence of PD device-assisted therapies (DATs) on the GM remains to be investigated.
Objectives
To profile acute gut microbial community alterations in response to PD DAT initiation.
Methods
Clinical data and stool samples were collected from 21 PD patients initiating either deep brain stimulation (DBS) or levodopa–carbidopa intestinal gel (LCIG) and ten spousal healthy control (HC) subjects. 16S amplicon sequencing of stool DNA enabled comparison of temporal GM stability between groups and with clinical measures, including disease alterations relative to therapy initiation.
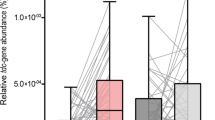
Results
We assessed GM response to therapy in the PD group by comparing pre-therapy (− 2 and 0 weeks) with post-therapy initiation timepoints (+ 2 and + 4 weeks) and HCs at baseline (0 weeks). Altered GM compositions were noted between the PD and HC groups at various taxonomic levels, including specific differences for DBS (overrepresentation of Clostridium_XlVa, Bilophila, Parabacteroides, Pseudoflavonifractor and underrepresentation of Dorea) and LCIG therapy (overrepresentation of Pseudoflavonifractor, Escherichia/Shigella, and underrepresentation of Gemmiger). Beta diversity changes were also found over the 4 week post-treatment initiation period.
Conclusions
We report on initial short-term GM changes in response to the initiation of PD DATs. Prior to the introduction of the DAT, a PD-associated GM was observed. Following initiation of DAT, several DAT-specific changes in GM composition were identified, suggesting DATs can influence the GM in PD.





Similar content being viewed by others
Availability of data and material
Upon request from authors.
Code availability
Custom code available at https://github.com/SydneyBioX/microbiome-PD
References
Lubomski M, Rushworth RL, Tisch S (2015) Hospitalisation and comorbidities in Parkinson’s disease: a large Australian retrospective study. J Neurol Neurosurg Psychiatry 86(3):324–330
Lubomski M, Davis RL, Sue CM (2020) Gastrointestinal dysfunction in Parkinson’s disease. J Neurol 267(5):1377–1388
Lubomski M, Davis RL, Sue CM (2020) Depression in Parkinson’s disease: perspectives from an Australian cohort. J Affect Disord 277:1038–1044
Lubomski M, Davis RL, Sue CM (2021) Health-related quality of life in Parkinson’s disease patients and their caregivers. J Mov Disord 14(1):42–52. https://doi.org/10.14802/jmd.20079
Hawkes CH, Del Tredici K, Braak H (2007) Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 33(6):599–614
Rietdijk CD, Perez-Pardo P, Garssen J, van Wezel RJ, Kraneveld AD (2017) Exploring Braak’s hypothesis of Parkinson’s disease. Front Neurol 8:37
Lim SY, Tan AH, Ahmad-Annuar A, Klein C, Tan LCS, Rosales RL et al (2019) Parkinson’s disease in the Western Pacific Region. Lancet Neurol 18(9):865–879
Antonini A, Moro E, Godeiro C, Reichmann H (2018) Medical and surgical management of advanced Parkinson’s disease. Mov Disord 33(6):900–908
Kruger R, Hilker R, Winkler C, Lorrain M, Hahne M, Redecker C et al (2016) Advanced stages of PD: interventional therapies and related patient-centered care. J Neural Transm 123(1):31–43
Wallen ZD, Appah M, Dean MN, Sesler CL, Factor SA, Molho E et al (2020) Characterizing dysbiosis of gut microbiome in PD: evidence for overabundance of opportunistic pathogens. NPJ Parkinson’s disease 6:11
van Kessel SP, Frye AK, El-Gendy AO, Castejon M, Keshavarzian A, van Dijk G et al (2019) Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat Commun 10(1):310
Maini Rekdal V, Bess EN, Bisanz JE, Turnbaugh PJ, Balskus EP (2019) Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science 364(6445):eaau6323. https://doi.org/10.1126/science.aau6323
Lubomski M, Davis RL, Sue CM (2019) The gut microbiota: a novel therapeutic target in Parkinson’s disease? Parkinsonism Relat Disord 66:265–266
Shreiner AB, Kao JY, Young VB (2015) The gut microbiome in health and in disease. Curr Opin Gastroenterol 31(1):69–75
Read MN, Holmes AJ (2017) Towards an integrative understanding of diet-host-gut microbiome interactions. Front Immunol 8:538
Lubomski M, Tan AH, Lim SY, Holmes AJ, Davis RL, Sue CM (2020) Parkinson’s disease and the gastrointestinal microbiome. J Neurol 267(9):2507–2523
Melis M, Vascellari S, Santoru ML, Oppo V, Fabbri M, Sarchioto M, Murgia D, Zibetti M, Lopiano L, Serra A, Palmas V, Pisanu S, Perra D, Madau V, Cusano R, Uva P, Mereu A, Contu P, Morelli M, Atzori L, Melis M, Manzin A, Cossu G (2021) Gut microbiota and metabolome distinctive features in Parkinson disease: focus on levodopa and levodopa-carbidopa intrajejunal gel. Eur J Neurol 28(4):1198–1209. https://doi.org/10.1111/ene.14644
Lewis SJ, Heaton KW (1997) Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 32(9):920–924
Barclay AW, Flood VM, Brand-Miller JC, Mitchell P (2008) Validity of carbohydrate, glycaemic index and glycaemic load data obtained using a semi-quantitative food-frequency questionnaire. Public Health Nutr 11(6):573–580
Palavra NC, Lubomski M, Flood VM, Davis RL, Sue CM (2021) Increased added sugar consumption is common in Parkinson’s disease. Front Nutr 8:207
Moayyedi P, Duffett S, Braunholtz D, Mason S, Richards ID, Dowell AC et al (1998) The Leeds Dyspepsia Questionnaire: a valid tool for measuring the presence and severity of dyspepsia. Aliment Pharmacol Ther 12(12):1257–1262
Sood R, Ford AC (2016) Diagnosis: Rome IV criteria for FGIDs—an improvement or more of the same? Nat Rev Gastroenterol Hepatol 13(9):501–502
Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD (1996) A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum 39(6):681–685
Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N (1997) The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing 26(5):353–357
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30(6):473–483
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–571
Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I et al (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699
McCormack HM, Horne DJ, Sheather S (1988) Clinical applications of visual analogue scales: a critical review. Psychol Med 18(4):1007–1019
Hagstromer M, Oja P, Sjostrom M (2006) The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr 9(6):755–762
Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P et al (2007) The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: results from an international pilot study. Mov Disord 22(13):1901–1911
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P et al (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23(15):2129–2170
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25(15):2649–2653
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173(2):697–703
Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M et al (2013) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic acids Res 41(1):e1
Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E et al (2015) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30(3):350–358
Heintz-Buschart A, Pandey U, Wicke T, Sixel-Doring F, Janzen A, Sittig-Wiegand E et al (2018) The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord 33(1):88–98
Barichella M, Severgnini M, Cilia R, Cassani E, Bolliri C, Caronni S, Ferri V, Cancello R, Ceccarani C, Faierman S, Pinelli G, De Bellis G, Zecca L, Cereda E, Consolandi C, Pezzoli G (2019) Unraveling gut microbiota in Parkinson's disease and atypical parkinsonism. Mov Disord 34(3):396–405. https://doi.org/10.1002/mds.27581
Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB et al (2015) Colonic bacterial composition in Parkinson’s disease. Mov Disord 30(10):1351–1360
Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Burmann J et al (2016) Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32:66–72
Bedarf JR, Hildebrand F, Coelho LP, Sunagawa S, Bahram M, Goeser F et al (2017) Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naive Parkinson’s disease patients. Genome medicine 9(1):39
Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD et al (2017) Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 32(5):739–749
Derrien M, Vaughan EE, Plugge CM, de Vos WM (2004) Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 54(5):1469–1476
Herath M, Hosie S, Bornstein JC, Franks AE, Hill-Yardin EL (2020) The role of the gastrointestinal mucus system in intestinal homeostasis: implications for neurological disorders. Front Cell Infect Microbiol 10:248
Cirstea MS, Yu AC, Golz E, Sundvick K, Kliger D, Radisavljevic N et al (2020) Microbiota composition and metabolism are associated with gut function in Parkinson’s disease. Mov Disord 35(7):1208–1217
Aho VTE, Pereira PAB, Voutilainen S, Paulin L, Pekkonen E, Auvinen P et al (2019) Gut microbiota in Parkinson’s disease: temporal stability and relations to disease progression. EBioMedicine 44:691–707
Minato T, Maeda T, Fujisawa Y, Tsuji H, Nomoto K, Ohno K et al (2017) Progression of Parkinson’s disease is associated with gut dysbiosis: two-year follow-up study. PLoS ONE 12(11):e0187307
D. Grün VZ, J. Kauffmann, M. Unger, J. Spiegel, KU. Dillmann, A. Schwiertz, K. Faßbender, M. Fousse. Impact of oral COMT-inhibitors on gut microbiota and biologically active microbial metabolites in patients with Parkinson’s Disease [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/impact-of-oral-comt-inhibitors-on-gut-microbiota-and-biologically-active-microbial-metabolites-in-patients-with-parkinsons-disease/. Accessed 20 Sept 2019.
Jin M, Li J, Liu F, Lyu N, Wang K, Wang L et al (2019) Analysis of the gut microflora in patients with Parkinson’s disease. Front Neurosci 13:1184
A. Hannoun DW, J. Flahive, J. Friedman, A. Deb, K. Smith. Effect of Levodopa on gut microbiome in Parkinson’s Disease (PD) [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/effect-of-levodopa-on-gut-microbiome-in-parkinsons-disease-pd/. Accessed 20 Sept 2019.
Parada Venegas D, De la Fuente MK, Landskron G, Gonzalez MJ, Quera R, Dijkstra G et al (2019) Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol 10:277
Bullich C, Keshavarzian A, Garssen J, Kraneveld A, Perez-Pardo P (2019) Gut vibes in Parkinson’s disease: the microbiota-gut-brain axis. Mov Disord Clin Pract 6(8):639–651
Dalile B, Van Oudenhove L, Vervliet B, Verbeke K (2019) The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol 16(8):461–478
Fasano A, Fung VSC, Lopiano L, Elibol B, Smolentseva IG, Seppi K et al (2019) Characterizing advanced Parkinson’s disease: OBSERVE-PD observational study results of 2615 patients. BMC Neurol 19(1):50
Hopfner F, Kunstner A, Muller SH, Kunzel S, Zeuner KE, Margraf NG et al (2017) Gut microbiota in Parkinson disease in a northern German cohort. Brain Res 1667:41–45
Lin A, Zheng W, He Y, Tang W, Wei X, He R et al (2018) Gut microbiota in patients with Parkinson’s disease in southern China. Parkinsonism Relat Disord 53:82–88
Perez-Cobas AE, Gosalbes MJ, Friedrichs A, Knecht H, Artacho A, Eismann K et al (2013) Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut 62(11):1591–1601
Bhalodi AA, van Engelen TSR, Virk HS, Wiersinga WJ (2019) Impact of antimicrobial therapy on the gut microbiome. J Antimicrob Chemother 74(Suppl 1):i6–i15
Freedman SN, Shahi SK, Mangalam AK (2018) The “Gut Feeling”: breaking down the role of gut microbiome in multiple sclerosis. Neurotherapeutics 15(1):109–125
Benjamin MM, Datta AR (1995) Acid tolerance of enterohemorrhagic Escherichia coli. Appl Environ Microbiol 61(4):1669–1672
Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J (2016) Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 65(1):57–62
Krygowska-Wajs A, Furgala A, Gorecka-Mazur A, Pietraszko W, Thor P, Potasz-Kulikowska K et al (2016) The effect of subthalamic deep brain stimulation on gastric motility in Parkinson’s disease. Parkinsonism Relat Disord 26:35–40
Acknowledgements
We thank Parkinson’s New South Wales for a Research Seed Grant to ML, CMS and RLD. Professor Vicki Flood and Mr Jon Flood for assistance analysing and interpreting the Food Frequency Questionnaire data. We would also like to sincerely thank all our participants for their patience and willingness to contribute to this research. ML is the recipient of a RACP Research Entry Scholarship and Northern Precinct Ramsay Scholarship. RLD was a New South Wales Health Early-Mid Career Research Fellow. CMS is a NHMRC Practitioner Fellow (APP1136800). This work is also supported by the Australian Research Council Discovery Project grant (DP170100654) for JYHY and XX.
Funding
Not industry sponsored. Supported by a Parkinson’s New South Wales, Research Seed Grant.
Author information
Authors and Affiliations
Contributions
ML: conceived and designed the study, recruited and examined all participants, collected, generated and analysed the data, drafted and reviewed the manuscript. XX: analysed the genomic and clinical data, drafted and reviewed the manuscript. AJH: designed the study, analysed data, drafted and reviewed the manuscript. JYHY: designed the genomic and clinical analysis, drafted and reviewed the manuscript. CMS: conceived and designed the study, drafted and reviewed the manuscript. RLD: conceived and designed the study, generated data, drafted and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
ML, XX, AJH, JYHY, CMS, RLD declare no conflicting/competing of interests.
Ethics approval
Ethical approval was granted by the Northern Sydney Local Health District Human Research Ethics Committee (HREC/18/HAWKE/109) and the North Shore Private Hospital ethics committee (NSPHEC 2018-LNR-009) and all participants provided written informed consent.
Consent to participate
All participants provided written informed consent.
Consent for publication
All authors provide consent for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lubomski, M., Xu, X., Holmes, A.J. et al. The impact of device-assisted therapies on the gut microbiome in Parkinson’s disease. J Neurol 269, 780–795 (2022). https://doi.org/10.1007/s00415-021-10657-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10657-9