Abstract
Background
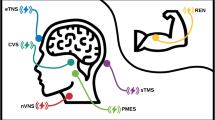
Implanted and transcutaneous nerve stimulators have shown promise as novel non-pharmacologic treatment for episodic and chronic migraines. The purpose of this study was to summarize the reported efficacy of transcutaneous single nerve stimulators in management of migraine frequency and severity.
Methods
A systematic review of five databases identified studies treating migraines with transcutaneous stimulation of a single nerve. Random effects model meta-analyses were conducted to establish the effect of preventive transcutaneous nerve stimulation on headache days per month and 0–10 numeric rating scale pain severity of headaches for both individuals with episodic and chronic migraines.
Results
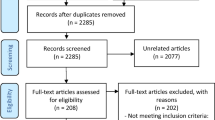
Fourteen studies, which treated 995 patients, met inclusion criteria, including 7 randomized controlled trials and 7 uncontrolled clinical trials. Transcutaneous nerve stimulators reduced headache frequency in episodic migraines (2.81 fewer headache days per month, 95% CI 2.18–3.43, I2 = 21%) and chronic migraines (2.97 fewer headache days per month, 95% CI 1.66–4.28, I2 = 0%). Transcutaneous nerve stimulators reduced headache severity in episodic headaches (2.23 fewer pain scale points, 95% CI 1.64–2.81, I2 = 88%).
Conclusions
Preventive use of transcutaneous nerve stimulators provided clinically significant reductions in headache frequency in individuals with chronic or episodic migraines. Individuals with episodic migraines also experienced a reduction in headache pain severity following preventive transcutaneous nerve stimulation.





Similar content being viewed by others
References
Stovner LJ, Nichols E, Steiner TJ et al (2018) Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 17(11):954–976. https://doi.org/10.1016/S1474-4422(18)30322-3
Steiner TJ, Stovner LJ, Jensen R et al (2020) Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain 21(1):137. https://doi.org/10.1186/s10194-020-01208-0
Burch R, Rizzoli P, Loder E (2018) The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache 58(4):496–505. https://doi.org/10.1111/head.13281
Vos T, Barber RM, Bell B et al (2015) Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386(9995):743–800. https://doi.org/10.1016/S0140-6736(15)60692-4
Stewart WF, Ricci JA, Chee E et al (2003) Lost productive time and cost due to common pain conditions in the US workforce. JAMA 290(18):2443–2454. https://doi.org/10.1001/jama.290.18.2443
Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. https://doi.org/10.1177/0333102417738202
Robbins MS (2021) Diagnosis and management of headache: a review. JAMA 325(18):1874–1885. https://doi.org/10.1001/jama.2021.1640
Filipović B, Matak I, Lacković Z (2014) Dural neurogenic inflammation induced by neuropathic pain is specific to cranial region. J Neural Transm 121(5):555–563. https://doi.org/10.1007/s00702-013-1144-4
Li C, White TG, Shah KA et al (2021) Percutaneous trigeminal nerve stimulation induces cerebral vasodilation in a dose-dependent manner. Neurosurgery 88(6):E529-e536. https://doi.org/10.1093/neuros/nyab053
Kosaras B, Jakubowski M, Kainz V et al (2009) Sensory innervation of the calvarial bones of the mouse. J Comp Neurol 515(3):331–348. https://doi.org/10.1002/cne.22049
Schueler M, Messlinger K, Dux M et al (2013) Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain 154(9):1622–1631. https://doi.org/10.1016/j.pain.2013.04.040
Goadsby PJ (2012) Pathophysiology of migraine. Ann Indian Acad Neurol 15(Suppl 1):S15–S22. https://doi.org/10.4103/0972-2327.99993
Tang Y, Kang J, Zhang Y et al (2017) Influence of greater occipital nerve block on pain severity in migraine patients: a systematic review and meta-analysis. Am J Emerg Med 35(11):1750–1754. https://doi.org/10.1016/j.ajem.2017.08.027
Seal RP (2016) Illuminating the gap: neuronal cross-talk within sensory ganglia and persistent pain. Neuron 91(5):950–951. https://doi.org/10.1016/j.neuron.2016.08.030
Piovesan EJ, Kowacs PA, Tatsui CE et al (2001) Referred pain after painful stimulation of the greater occipital nerve in humans: evidence of convergence of cervical afferences on trigeminal nuclei. Cephalalgia 21(2):107–109. https://doi.org/10.1046/j.1468-2982.2001.00166.x
Busch V, Jakob W, Juergens T et al (2006) Functional connectivity between trigeminal and occipital nerves revealed by occipital nerve blockade and nociceptive blink reflexes. Cephalalgia 26(1):50–55. https://doi.org/10.1111/j.1468-2982.2005.00992.x
Chua NH, van Suijlekom HA, Vissers KC et al (2011) Differences in sensory processing between chronic cervical zygapophysial joint pain patients with and without cervicogenic headache. Cephalalgia 31(8):953–963. https://doi.org/10.1177/0333102411408358
Dodick DW, Silberstein SD, Reed KL et al (2015) Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: long-term results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 35(4):344–358. https://doi.org/10.1177/0333102414543331
Silberstein SD, Dodick DW, Saper J et al (2012) Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 32(16):1165–1179. https://doi.org/10.1177/0333102412462642
Bono F, Salvino D, Mazza M et al (2015) The influence of ictal cutaneous allodynia on the response to occipital transcutaneous electrical stimulation in chronic migraine and chronic tension-type headache: a randomized, sham-controlled study. Cephalalgia 35(5):389–398. https://doi.org/10.1177/0333102414544909
Birlea M, Penning S, Callahan K et al (2019) Efficacy and safety of external trigeminal neurostimulation in the prevention of chronic migraine: an open-label trial. Cephalalgia Rep 2:2515816319856625. https://doi.org/10.1177/2515816319856625
Chou DE, Gross GJ, Casadei CH et al (2017) External trigeminal nerve stimulation for the acute treatment of migraine: open-label trial on safety and efficacy. Neuromodul: Technol Neural Interface 20(7):678–683. https://doi.org/10.1111/ner.12623
Chou DE, Shnayderman Yugrakh M, Winegarner D et al (2019) Acute migraine therapy with external trigeminal neurostimulation (ACME): a randomized controlled trial. Cephalalgia 39(1):3–14. https://doi.org/10.1177/0333102418811573
Deng Y, Zheng M, He L et al (2020) A head-to-head comparison of percutaneous mastoid electrical stimulator and supraorbital transcutaneous stimulator in the prevention of migraine: a prospective, randomized controlled study. Neuromodul: Technol Neural Interface 23(6):770–777. https://doi.org/10.1111/ner.13127
Diener H-C, Goadsby PJ, Ashina M et al (2019) Non-invasive vagus nerve stimulation (nVNS) for the preventive treatment of episodic migraine: the multicentre, double-blind, randomised, sham-controlled PREMIUM trial. Cephalalgia 39(12):1475–1487. https://doi.org/10.1177/0333102419876920
Hokenek NM, Erdogan MO, Hokenek UD et al (2021) Treatment of migraine attacks by transcutaneous electrical nerve stimulation in emergency department: a randomize controlled trial. Am J Emerg Med 39:80–85. https://doi.org/10.1016/j.ajem.2020.01.024
Kinfe T, Pintea B, Roeske S et al (2016) Percutaneous nerve field stimulation (PENS) of the occipital region as a possible predictor for occipital nerve stimulation (ONS) responsiveness in refractory headache disorders? A feasibility study. Cephalalgia 36(8):779–789. https://doi.org/10.1177/0333102415613765
Kinfe TM, Pintea B, Muhammad S et al (2015) Cervical non-invasive vagus nerve stimulation (nVNS) for preventive and acute treatment of episodic and chronic migraine and migraine-associated sleep disturbance: preliminary findings from a prospective observational cohort study. J Headache Pain 16(1):101. https://doi.org/10.1186/s10194-015-0582-9
Liu Y, Dong Z, Wang R et al (2017) Migraine prevention using different frequencies of transcutaneous occipital nerve stimulation: a randomized controlled trial. J Pain 18(8):1006–1015. https://doi.org/10.1016/j.jpain.2017.03.012
Nguyen J-P, Nizard J, Kuhn E et al (2016) A good preoperative response to transcutaneous electrical nerve stimulation predicts a better therapeutic effect of implanted occipital nerve stimulation in pharmacologically intractable headaches. Neurophysiologie Clinique/Clin Neurophysiol 46(1):69–75. https://doi.org/10.1016/j.neucli.2015.12.002
Ordás CM, Cuadrado ML, Pareja JA et al (2020) Transcutaneous supraorbital stimulation as a preventive treatment for chronic migraine: a prospective, open-label study. Pain Med 21(2):415–422. https://doi.org/10.1093/pm/pnz119
Przeklasa-Muszyńska A, Skrzypiec K, Kocot-Kępska M et al (2017) Non-invasive transcutaneous Supraorbital Neurostimulation (tSNS) using Cefaly® device in prevention of primary headaches. Neurol Neurochir Pol 51(2):127–134. https://doi.org/10.1016/j.pjnns.2017.01.004
Russo A, Tessitore A, Conte F et al (2015) Transcutaneous supraorbital neurostimulation in “de novo” patients with migraine without aura: the first Italian experience. J Headache Pain 16(1):69. https://doi.org/10.1186/s10194-015-0551-3
Schoenen J, Vandersmissen B, Jeangette S et al (2013) Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology 80(8):697–704. https://doi.org/10.1212/WNL.0b013e3182825055
Silberstein SD, Calhoun AH, Lipton RB et al (2016) Chronic migraine headache prevention with noninvasive vagus nerve stimulation. The EVENT study. Neurology 87(5):529–538. https://doi.org/10.1212/wnl.0000000000002918
Straube A, Ellrich J, Eren O et al (2015) Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t-VNS): a randomized, monocentric clinical trial. J Headache Pain 16(1):63. https://doi.org/10.1186/s10194-015-0543-3
Tassorelli C, Grazzi L, de Tommaso M et al (2018) Noninvasive vagus nerve stimulation as acute therapy for migraine: The randomized PRESTO study. Neurology 91(4):e364–e373. https://doi.org/10.1212/wnl.0000000000005857
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. https://doi.org/10.1136/bmj.b2535
Higgins JPT, Thomas J, Chandler J et al (2021) Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane. Available from https://www.training.cochrane.org/handbook
McCoy CE (2017) Understanding the intention-to-treat principle in randomized controlled trials. West J Emerg Med 18(6):1075–1078. https://doi.org/10.5811/westjem.2017.8.35985
Higgins J, Greene S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. In: 7.7.7.2 Obtaining standard errors from confidence intervals. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6294583/2011
Furukawa TA, Barbui C, Cipriani A et al (2006) Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 59(1):7–10. https://doi.org/10.1016/j.jclinepi.2005.06.006
Lee YH (2018) An overview of meta-analysis for clinicians. Korean J Intern Med 33(2):277–283. https://doi.org/10.3904/kjim.2016.195
McKenzie JE, Herbison GP, Deeks JJ (2016) Impact of analysing continuous outcomes using final values, change scores and analysis of covariance on the performance of meta-analytic methods: a simulation study. Res Syn Methods 7(4):371–386. https://doi.org/10.1002/jrsm.1196
Olsen MF, Bjerre E, Hansen MD et al (2017) Pain relief that matters to patients: systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med 15(1):35. https://doi.org/10.1186/s12916-016-0775-3
Silberstein SD, Marmura MJ, Shaw J et al (2010) Headache prophylaxis with BoNTA: patient characteristics. Headache: J Head Face Pain 50(1):63–70. https://doi.org/10.1111/j.1526-4610.2009.01481.x
Danno D, Iigaya M, Imai N et al (2019) The safety and preventive effects of a supraorbital transcutaneous stimulator in Japanese migraine patients. Sci Rep 9(1):9900. https://doi.org/10.1038/s41598-019-46044-8
Magis D, D’Ostilio K, Thibaut A et al (2017) Cerebral metabolism before and after external trigeminal nerve stimulation in episodic migraine. Cephalalgia 37(9):881–891. https://doi.org/10.1177/0333102416656118
Cefaly Dual K173006 approval letter (2017) U.S. Food and Drug Administration. https://www.accessdata.fda.gov/cdrh_docs/pdf17/K173006.pdf. Accessed 15 Dec 2021
Burstein R, Blumenfeld AM, Silberstein SD et al (2020) Mechanism of action of onabotulinumtoxina in chronic migraine: a narrative review. Headache 60(7):1259–1272. https://doi.org/10.1111/head.13849
Tao H, Wang T, Dong X et al (2018) Effectiveness of transcutaneous electrical nerve stimulation for the treatment of migraine: a meta-analysis of randomized controlled trials. J Headache Pain 19(1):42–42. https://doi.org/10.1186/s10194-018-0868-9
Halker Singh RB, Ailani J, Robbins MS (2019) Neuromodulation for the acute and preventive therapy of migraine and cluster headache. Headache 59(Suppl 2):33–49. https://doi.org/10.1111/head.13586
Migraine: Developing Drugs for Acute Treatment Guidance for Industry (2018) Food and Drug Administration Center for Drug Evaluation and Research. https://www.fda.gov/media/89829/download. Accessed 14 Feb 2022
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Ethics approval
This is a review of published literature and did not involve human subjects. An approval by an ethics committee was not applicable.
Informed consent
This is a review of published literature and did not involve human subjects.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Evans, A.G., Horrar, A.N., Ibrahim, M.M. et al. Outcomes of transcutaneous nerve stimulation for migraine headaches: a systematic review and meta-analysis. J Neurol 269, 4021–4029 (2022). https://doi.org/10.1007/s00415-022-11059-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11059-1