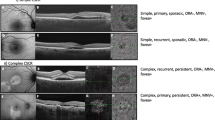
Abstract
Purpose
Validation of a recently described central serous chorioretinopathy (CSCR) classification system and assessment of levels of agreement among 10 retina physicians.
Methods
This was a cross-sectional (inter-reader agreement) study. Ten retina physicians (assigned a role of masked grader) were provided with a comprehensive dataset of 61 eyes of 34 patients of presumed CSCR. Relevant clinical details and multimodal imaging (fundus autofluorescence, fluorescein and indocyanine green angiography, optical coherence tomography) of both involved and fellow eye were electronically shared. Later, only the fellow eye images were resent to understand the influence of affected eye on the grading of the fellow eye. Multiple inter-grader agreement using Fleiss Kappa was performed to determine the level of agreement among the 10 graders. p value of ≤ 0.05 was considered statistically significant.
Results
Sixty-one eyes of 34 patients were evaluated. There was moderate agreement for major criteria with Fleiss Kappa value of 0.50 (p < 0.0001) with a single outlier observer. After excluding that observer, the Fleiss Kappa value increased to 0.57 (p < 0.0001) with statistically significant p values among all categories, i.e., simple CSC (\(\kappa\) = 0.575), complex CSC (\(\kappa\) = 0.621), and no CSC (\(\kappa\) = 0.452). Overall, moderate to substantial agreement was noted among the subtypes (primary, recurrent, and resolved). The influence of the affected eye on fellow eye grading was studied. The global Fleiss Kappa coefficient (\(\kappa\) = 0.642, p < 0.0001) showed substantial agreement when observers were aware of the affected eye grading. However, without prior available information on the affected eye, the inter-grader agreement was significantly lower (global \(\kappa\) = 0.255, p < 0.0001).
Conclusion
A fair-moderate inter-grader agreement among the masked graders suggests a need for further refinement of this novel classification system. Disease grading should include both eyes as lack of information on affected eye has a bearing on fellow eye grading and inter-grader agreement as shown by a significant difference in global \(\kappa\) values.





Similar content being viewed by others
Availability of data and material
Available upon request.
Code availability
Available upon request.
Change history
06 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00417-021-05484-7
References
Daruich A, Matet A, Dirani A et al (2015) Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog Retin Eye Res 48:82–118
Balaratnasingam C, Freund KB, Tan AM et al (2016) Bullous Variant of Central Serous Chorioretinopathy: Expansion of Phenotypic Features Using Multimethod Imaging. Ophthalmology 123(7):1541–1552
Mrejen S, Balaratnasingam C, Kaden TR et al (2019) Long-term Visual Outcomes and Causes of Vision Loss in Chronic Central Serous Chorioretinopathy. Ophthalmology 126(4):576–588
Nicholson B, Noble J, Forooghian F et al (2013) Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol 58(2):103–126
Singh SR, Matet A, van Dijk EHC et al (2019) Discrepancy in current central serous chorioretinopathy classification. Br J Ophthalmol 103(6):737–742
Chhablani J, Cohen FB (2020) Multimodal Imaging-Based Central Serous Chorioretinopathy Classification. Ophthalmol Retina 4(11):1043–1046
Fleiss JL (1971) Measuring nominal scale agreement among many raters. Psychol Bull 76(5):378–382
Jabs DA, Dick A, Doucette JT et al (2018) Interobserver Agreement Among Uveitis Experts on Uveitic Diagnoses: The Standardization of Uveitis Nomenclature Experience. Am J Ophthalmol 186:19–24
Chandra S, Rasheed R, Sen P et al (2021) Inter-rater reliability for diagnosis of geographic atrophy using spectral domain OCT in age-related macular degeneration. Eye. https://doi.org/10.1038/s41433-021-01490-5
Arora S, Kulikov AN, Maltsev DS (2021) Implementation of the new multimodal imaging-based classification of central serous chorioretinopathy. Eur J Ophthalmol. https://doi.org/10.1177/11206721211013651
Ng DS, Ho M, Chen LJ et al (2021) Optical Coherence Tomography Angiography Compared with Multimodal Imaging for Diagnosing Neovascular Central Serous Chorioretinopathy. Am J Ophthalmol. https://doi.org/10.1016/j.ajo.2021.05.029
van Dijk EHC, Boon CJF (2021) Serous business: Delineating the broad spectrum of diseases with subretinal fluid in the macula. Prog Retin Eye Res 84:100955
van Rijssen TJ, van Dijk EHC, Yzer S et al (2019) Central serous chorioretinopathy: Towards an evidence-based treatment guideline. Prog Retin Eye Res 73:100770
Acknowledgements
CSCR International Group
Affiliations
1. Pauline Aymard, Ophtalmopole, Cochin Hospital, University of Paris, France
2. Talal Beydoun, Ophtalmopole, Cochin Hospital, University of Paris, France
3. Elodie Bousquet, Ophtalmopole, Cochin Hospital, University of Paris, France
4. Francine Behar-Cohen, Ophtalmopole, Cochin Hospital, University of Paris, France
5. Chadi Mehanna, Ophtalmopole, Cochin Hospital, University of Paris, France
6. Jay Chhablani, Department of Ophthalmology, University of Pittsburgh Eye and Ear Institute, Pittsburgh, PA. USA
7. Chui Ming Gemmy Cheung, Singapore Eye Research Institute, Singapore National Eye Centre, Singapore, Singapore, Duke-NUS Medical School, National University of Singapore, Singapore, Singapore.
8. Alejandra Daruich, Ophtalmopole, Cochin Hospital, University of Paris, France
9. K. Bailey Freund, Vitreous Retina Macula Consultants Of New York, New York, New York; Department of Ophthalmology, NYU School of Medicine, New York, New York, USA
10. Alain Gaudric, Université De Paris, Hopital Lariboisière, 2 Rue Ambroise Paré, 75,010 Paris, France
11. Camiel J.F. Boon, Department of Ophthalmology, Leiden University Medical Center, the Netherlands; Department of Ophthalmology, Amsterdam University Medical Centers, the Netherlands
12. Andrew Lotery, Faculty of Medicine, University Of Southampton, England.
13. Marco Lupidi, Department of Biochemical and Surgical Sciences, Section of Ophthalmology, University of Perugia, Perugia, Italy
14. Irmela Mantel, Department of Ophthalmology, University of Lausanne, Jules Gonin Eye Hospital, Foundation Asile des Aveugles, 15 Avenue de France, CP 5143, CH-1000, Lausanne, Switzerland.
15. Thibaud Mathis, Croix-Rousse University Hospital, Hospices Civils de Lyon, UMR-CNRS 5510 Matéis, Lyon 1 University, France
16. Alexandre Matet, Ophtalmopole, Cochin Hospital, University of Paris, France
17. Martine Mauget-Faÿsse, Clinical Investigative Platform, Rothschild Foundation, 75,019 Paris, France
18. Sarah Mrejen, Department of Ophthalmology, XV-XX Ophthalmology National Hospital Center, Paris, France. Centre d'Imagerie et de Laser, 11 rue Antoine Bourdelle, 75,015, Paris, France.
19. Giuseppe Querques, School of Medicine, Vita-Salute San Raffaele University, Via Olgettina, 58, 20,132 Milan, Italy; Division of head and neck, Ophthalmology Unit, IRCCS San Raffaele Scientific Institute, Via Olgettina, 60, 20,132 Milan, Italy
20. Jorge Ruiz-Medrano, Puerta De Hierro-Majadahonda University Hospital, Madrid, Spain
21. Jose-Maria Ruiz-Moreno, Puerta De Hierro-Majadahonda University Hospital, Madrid, Spain
22. Shiri Shulman, Assuta Medical Centers, Tel Aviv, Israël
23. Sumit Randhir Singh, Jacobs Retina Center at Shiley Eye Center, University of California, San Diego, La Jolla, CA, USA
24. Sobha Sivaprasad, NIHR Moorfields Biomedical Research Centre, Moorfields Eye Hospital, 162 City Road, London, EC1V2PD, UK
25. Richard F. Spaide, Vitreous Retina Macula Consultants Of New York, New York, New York, USA
26. Elon.H.C van Dijk, Department of Ophthalmology, Leiden University Medical Center, the Netherlands
27. Suzanne Yzer, Rotterdam Eye Hospital, Rotterdam, The Netherlands.
28. Min Zhao, Ophtalmopole, Cochin Hospital, University of Paris, France
29. Sandrine Zweifel, Department of Ophthalmology, University Hospital Zurich, Frauenklinikstrasse 24, 8091 Zurich, Switzerland And University of Zurich, Zurich, Switzerland
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was waived by the local Ethics Committee of University of Pittsburgh in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised. The individual collaborator names under the group name “CSCR International Group” are now added.
Rights and permissions
About this article
Cite this article
Chhablani, J., Behar-Cohen, F. & Central Serous Chorioretinopathy International Group. Validation of central serous chorioretinopathy multimodal imaging-based classification system. Graefes Arch Clin Exp Ophthalmol 260, 1161–1169 (2022). https://doi.org/10.1007/s00417-021-05452-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05452-1