Abstract
Purpose
Acute acalculous cholecystitis (AAC) is characterized by severe gallbladder inflammation without cystic duct obstruction. Critical illness and neurological deficits are often associated with AAC, and early radiologic imaging is necessary for the detection and timely treatment of AAC. In critically ill patients, effective surgical management is difficult. This review examines the three common surgical treatments for AAC (open cholecystectomy (OC), laparoscopic cholecystectomy (LC), or percutaneous cholecystostomy (PC)), their prevalence in current literature, and the perioperative outcomes of these different approaches using a large retrospective database.
Materials and methods
This review examined literature gathered from PubMed and Google Scholar to select more than 50 sources with data pertinent to AAC; of which 20 are described in a summary table. Outcomes from our previous research and several updated results were obtained from the University HealthSystem Consortium (UHC) database.
Results
LC has proven effective in treating AAC when the risks of general anesthesia and the chance for conversion to OC are low. In critically ill patients with multiple comorbidities, PC or OC may be the only available options. Data in the literature and an examination of outcomes within a national database indicate that for severely ill patients, PC may be safer and met with better outcomes than OC for the healthier set of AAC patients.
Conclusions
We suggest a three-pronged approach to surgical resolution of AAC. Patients that are healthy enough to tolerate LC should undergo LC early in the course of the disease. In critically ill patients, patients with multiple comorbidities, a high conversion risk, or who are poor surgical candidates, PC may be the safest and most successful intervention.
Similar content being viewed by others
Introduction
Acute acalculous cholecystitis (AAC) is a disease characterized by severe gallbladder inflammation without cystic duct obstruction due to gallstones. First recognized in 1844, AAC accounts for 2–15 % of acute cholecystitis cases [1–5]. AAC is associated with considerably higher patient morbidity as well as a mortality rate of up to 50 % [6–8] and typically has a worse prognosis than its calculous counterpart (ACC) [3, 9, 10].
Due to the severity of illness and nonspecific presentation, a high index of suspicion and early radiologic imaging are necessary to detect AAC [1, 2, 9, 11–14]. Critical illness and neurological deficits are frequently associated with AAC and may hinder an expedient diagnosis [3, 8]. AAC may be suspected with right upper quadrant pain and tenderness, an enlarged gallbladder, and a positive ultrasonographic Murphy’s sign. Radiologic confirmation of a distended gallbladder, thickened wall, and pericholecystic fluid further suggest AAC [3, 11, 14, 15]. An absence of gallstones observed in imaging or laparoscopy confirms a diagnosis of AAC [11]. Complications of AAC are life-threatening and include gallbladder perforation, gangrene, empyema, and sepsis [4, 9, 11, 12, 16–18].
AAC occurs most frequently in critically ill patients, with incidence in this category ranging from 0.5 to 18 % [6]. AAC typically complicates surgery and can occur in conjunction with multiple organ failure, burn injury, or major trauma [1, 4, 7, 10, 13, 17, 19–21]. AAC also may coincide with congestive heart failure, diabetes mellitus, embolization of the cystic artery, immunosuppression, and abdominal vasculitis [14, 17, 22, 23]. In these patients, AAC often represents further progression of multiple systemic failure [2, 7]. This necessitates immediate action with the most appropriate and clinically successful surgical intervention possible. While some sources report that AAC is not limited to the critically ill [24–26], these cases remain outside the scope of this review.
AAC treatment options include open or laparoscopic cholecystectomy (OC and LC) or percutaneous cholecystostomy (PC). Open cholecystectomy originally served as the primary method of treatment [27], but the advent of minimally invasive techniques has enabled these operations to be performed laparoscopically [28]; some physicians instead opt for percutaneous drainage in cases of AAC [12]. In these critically ill patients, effective surgical management is difficult. Borzellino et al. notes the need for a comprehensive study of treatment modalities in the critically ill [29]. Discrepancies exists as to the preferred method of treatment [30, 31], and we believe that a large population-based study will help to determine the most successful intervention. In this review, we examine OC, LC, and PC and their clinical outcomes in the literature and using data from University HealthSystem Consortium (UHC).
Treatment options
Open cholecystectomy
OC is the traditional surgical intervention in cases of acute acalculous cholecystitis. Open removal of the gallbladder provides a definitive solution to cholecystitis, eliminating the possibility of recurrence [27, 32–36]. OC also allows easier surgical management of unclear anatomy, bleeding, and complications [27, 37]; when these complications manifest in LC and PC, the operation is typically converted to OC [15, 38]. Some surgeons perform only OC because they prefer immediate, aggressive action against AAC [34, 36]; they posit that OC improves cases of multiorgan failure, particularly restoring cardiovascular and respiratory function [34]. AAC complicated by gallbladder ischemia or a gangrenous wall also necessitates open cholecystectomy [34].
Current literature suggests several weaknesses of OC. The critically ill patient diagnosed with AAC is an unsafe candidate for surgery under general anesthesia [2, 32, 39–42]. In these patients, OC consistently results in high mortality and morbidity as compared to other methods [23, 30, 39, 40, 43]. Chung et al. cite 63–100 % morbidity and 31–57 % mortality as a result of emergent abdominal surgery in critically ill elderly patients and encountered 19 % mortality with OC as the primary treatment. OC elsewhere may be associated with up to 44–59 % mortality [2, 7, 41, 44]. Orlando et al. asserts that OC should be considered only in the case of severe gallbladder necrosis. Simorov et al. states that even in healthier patients, OC still met with worse outcomes than PC.
Laparoscopic cholecystectomy
LC serves as an effective substitute for open surgery. Less invasive than OC, LC is often preferred in cases indicating cholecystectomy. LC includes many advantages of OC, including definitive resolution and prevention of future gallbladder pathology [12, 45, 46]. LC carries distinct benefits from OC, such as decreased rate of infection, mortality, length of stay, and cost [4, 12, 15, 28, 38, 45, 47, 48]. Laparoscopy can be both diagnostic and therapeutic, which is useful in confirming a commonly elusive diagnosis such as AAC [6, 49–51]. Patient satisfaction with LC is relatively high [52, 53] as length of stay, recovery time, and cosmetic results are significantly improved when compared to the open procedure [15, 37, 52, 53]. Early LC is highly successful in treating AAC [15, 40, 54].
The disadvantages of laparoscopic cholecystectomy should be considered. First, a laparoscopic approach may not be possible for all surgeons. LC also requires general anesthesia, and this procedure is known to be of a higher risk in the critically ill [2, 32, 39–42, 55]. Additionally, converting to an open procedure poses a significant risk of complication in those with multiple comorbidities; literature suggests that conversion rates from LC to OC may be as high as 20–35 %, with men and obese patients most susceptible to conversion [30, 38, 40, 46, 56–58]. Conversion to OC increases operative cost and complication rate in addition to losing the benefit of the laparoscopic approach [41]. LC is frequently performed only on the most fit AAC patients and may be unsuited for the severely ill.
Percutaneous cholecystostomy
PC has proven safe, rapid, and highly efficacious in treating acute acalculous cholecystitis. As a conservative treatment option, PC can be performed at the bedside under local anesthesia. PC demonstrates a significant decrease in complication rate and consequent lower patient morbidity and mortality than OC and LC [30, 40, 59, 60]. The cholecystostomy tube is placed transhepatically with radiologic guidance, and drains the acutely inflamed gallbladder; it typically remains for 3 weeks or until subsequent cholecystectomy [12, 39, 40, 59, 61, 62]. Patient fitness for general anesthesia and surgery must be considered because AAC most commonly occurs in the critically ill. In patients too ill to undergo the open or laparoscopic procedure, minimally invasive management by therapeutic drainage serves as a potentially lifesaving treatment [1, 3, 6, 33, 41, 44, 63–66]. PC optimizes the patient’s condition by normalizing local symptoms as well as reducing the overall inflammatory response [63, 64, 67]. Additionally, when PC is performed as a first response to AAC diagnosis, some patients may not require subsequent cholecystectomy. After drainage alone, patient improvement may be significant enough not to warrant further operation [6, 12, 39, 42, 44, 55, 59–61, 66, 68–73]. Chung et al. demonstrated a 93 % rate of symptom resolution in critically ill AAC patients.
It is also important to examine the weaknesses of percutaneous cholecystostomy. In many cases, it serves as only temporary relief until cholecystectomy may be safely performed. Even when PC successfully resolves symptoms, the gallbladder remains and is still susceptible to recurrence [32, 44]. Because cholecystostomy may not be a permanent resolution, many consider open or laparoscopic cholecystectomy the standard AAC treatment [32, 45, 49, 74]. Cholecystostomy is contraindicated in cases of gangrene or gallbladder perforation [6, 34], which may have an incidence from 37 to 81 % in AAC [4]. The cholecystostomy tube also inherently carries the risk of causing complication or infection, though this is quite rare [44]. In a large-scale population study, Anderson et al. posits that PC poses no significant benefit in cases of AAC, citing a higher mortality rate than no intervention at all [20, 75].
Materials and methods
Literature review of articles with data describing outcomes of OC, LC, and PC identified 50 sources containing information of interest. Searches were conducted using Google Scholar and PubMed. Of the 50 sources, 20 were selected based on their content specific to AAC for inclusion in the summary table included in this paper.
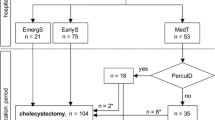
Recent data on AAC patients was gathered from the UHC database to determine if the findings presented previously by us and others are still supported in the overall population of patients receiving these procedures. UHC is an alliance of 100 academic medical centers and 250 of their affiliated hospitals, representing 90 % of the nation’s nonprofit academic medical centers. The information available through the UHC database includes that on the patient discharge, inpatient hospital stay, patient characteristics, length of stay (LOS), 30-day readmission rate, postoperative morbidity, risk-adjusted in-hospital mortality, and inpatient care costs [76, 77]. Patients were selected using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis (575.0, 575.1) and procedure codes (OC: 51.22, LC: 51.23, PC: 51.01). Data on deaths occurring after discharge are not included in the UHC database. Readmission is defined as readmission for any reason within 30 days of discharge after the index procedure. LOS in the database is the period from the index procedure to the hospital discharge. This cost of patient care provided in the database is an estimated cost using a ratio of cost to charge.
Results
The literature
A systematic literature review yielded the results from a variety of studies on surgical interventions for AAC. These studies addressed the benefits and disadvantages of treatment, many of them explicitly comparing interventions. Table 1 summarizes the discrepancies between treatment options while illustrating a clear gap in current literature—the need for a large-scale, objective comparison of OC, LC, and PC (Table 1).
Our supplemental findings
Analysis conducted by our researchers in 2013 [40] concluded that PC was superior in its outcomes to LC and OC in severely ill patients. Similar relationships can be seen in updated analyses of all severity patient comparisons of PC vs OC from the same data source (Table 2). In the current results, morbidity occurred in 11.3 % of patients with PC compared to 14.2 % that received OC (p < 0.05) while mortality was also lower in the PC group (11.3 % vs 14.2 %, p < 0.05). Median length of stay was also a single day shorter in PC vs OC, similar to previous results. While readmission in our earlier study favored LC and OC, the current PC vs OC comparison demonstrates no significant difference in LOS between groups (Table 2).
Discussion
AAC in the critically ill patient presents significant difficulties in clinical management. Multiple surgical options exist, and the severity of AAC warrants an evidence-based recommendation.
In this review, we examined the three common AAC treatment methods, their prevalence in current literature, and their perioperative outcomes. As many studies indicate, LC has been proven effective in treating acute cholecystitis [4, 12, 28, 45, 46, 48, 56]. The high conversion rate, however, represents a significant increase in patient morbidity and mortality. In stable AAC patients, when the risks under general anesthesia and for conversion to OC are low, we agree that a laparoscopic approach should be the preferred surgical intervention. However, in critically ill patients with multiple comorbidities, only PC or OC may be available to surgeons [64, 65]. In these patients, our review indicates that PC is superior to OC and converted LC. Even in more severely ill patients, PC was safer and met with better outcomes than the outcomes observed in healthier OC patients [40]. As illness and liability of conversion increase, especially in critically ill patients unfit for laparoscopy, we strongly recommend PC as a bridging or definitive procedure.
Several studies indicate that PC may be a definitive treatment for AAC without requiring subsequent cholecystectomy [31, 37]. These patients improve significantly with PC alone, and further intervention may not be required after the removal of the cholecystostomy tube and resolution of the initial contributing condition.
With regard to our current findings, we suggest a threefold approach to surgical resolution of AAC. Patients healthy enough to tolerate it should undergo LC early in the course of illness [28, 48, 56]. In critically ill patients as well as patients with multiple comorbidities, high conversion risk, or poor surgical candidates, PC represents the safest and most successful intervention. These patients may later require cholecystectomy and should be evaluated for further treatment as their condition improves [31, 37, 46, 54, 64, 65]. Anderson et al. critique the role of PC with regard to OC, but largely analyze two different populations; their PC group had a sevenfold incidence of severe sepsis and shock as well as higher comorbidity compared to their OC patients. While they raise important questions regarding AAC care, we believe these factors to be significant.
Our previous study and updated figures are limited by factors inherent to any large, multicenter administrative database [40]. As a retrospective study, patient randomization was not possible. Aggregate data was collected and current database limitations inhibit patient-level outcomes including operative time, postoperative analgesia requirement, morbidity after the 30-day postoperative period, and quality-of-life assessments. These measures will likely be examined in the future and are important in evaluating clinical outcomes.
Conclusions
When examining the body of literature on the topic, we conclude that PC provides better outcomes with lower cost in AAC patients. Those at low risk for conversion and medically suited for the operation should be treated with LC. However, in high-risk patients unfit for surgery under general anesthesia, management by cholecystectomy poses a significant health threat. Even in sicker patients, PC has superior perioperative outcomes than OC. PC serves as a safe, highly successful, and cost-effective treatment of AAC whether as definitive treatment or a bridge to further intervention. Our expanded reanalysis of PC and OC supports these findings, and we therefore recommend physicians consider PC in patients with AAC.
References
Long TN, Heimbach DM, Carrico CJ (1978) Acalculous cholecystitis in critically III patients. Am J Surg 136:31–36. doi:10.1016/0002-9610(78)90196-4
Orlando R 3rd, Gleason E, Drezner AD (1983) Acute acalculous cholecystitis in the critically ill patient. Am J Surg 145:472–476
Gu MG, Kim TN, Song J, Nam YJ, Lee JY, Park JS (2014) Risk factors and therapeutic outcomes of acute acalculous cholecystitis. Digestion 90:75–80. doi:10.1159/000362444
Nikfarjam M, Manya K, Fink MA, Hadj AK, Muralidharan V, Starkey G et al (2012) Outcomes of patients with histologically proven acute acalculous cholecystitis. ANZ J Surg 82:918–922. doi:10.1111/j.1445-2197.2012.06202.x
Tsai YM, Yu JC, Chen CJ, Chan DC, Chang H, Lee SC et al (2011) Clinical pitfall and challenge: acute acalculous cholecystitis in a critically ill traumatic patient in the intensive care unit. J Med Sci 31:289–291
Yang HK, Hodgson WJ (1996) Laparoscopic cholecystostomy for acute acalculous cholecystitis. Surg Endosc 10:673–675
Laurila J, Syrjala H, Laurila PA, Saarnio J, Ala-Kokko TI (2004) Acute acalculous cholecystitis in critically ill patients. Acta Anaesthesiol Scand 48:986–991. doi:10.1111/j.0001-5172.2004.00426.x
Frazee RC, Nagorney DM, Mucha P Jr (1989) Acute acalculous cholecystitis. Mayo Clin Proc 64:163–167
Kalliafas S, Ziegler DW, Flancbaum L, Choban PS (1998) Acute acalculous cholecystitis: incidence, risk factors, diagnosis, and outcome. Am Surg 64:471–475
Jönsson P (1976) Postoperative acute acalculous cholecystitis. Arch Surg 111:1097. doi:10.1001/archsurg.1976.01360280055008
Liu FL, Li H, Wang XF, Shen KT, Shen ZB, Sun YH et al (2014) Acute acalculous cholecystitis immediately after gastric operation: case report and literatures review. World J Gastroenterol 20:10642–10650. doi:10.3748/wjg.v20.i30.10642
Huffman JL, Schenker S (2010) Acute acalculous cholecystitis: a review. Clin Gastroenterol Hepatol 8:15–22. doi:10.1016/j.cgh.2009.08.034
Ahmed N (2008) Acute acalculous cholecystitis complicating major trauma: a report of five cases. South Med J 101:1146–1149. doi:10.1097/SMJ.0b013e318181d587
Wang AJ, Wang TE, Lin CC, Lin SC, Shih SC (2003) Clinical predictors of severe gallbladder complications in acute acalculous cholecystitis. World J Gastroenterol 9:2821–2823
Pessaux P, Tuech JJ, Rouge C, Duplessis R, Cervi C, Arnaud JP (2000) Laparoscopic cholecystectomy in acute cholecystitis. A prospective comparative study in patients with acute vs. chronic cholecystitis. Surg Endosc 14:358–361. doi:10.1007/s004640020088
Srinivasan D, Thimmappa D, Sherigar S (2014) Gall bladder perforation in acalculous cholecystitis. Oncol Gastroenterol Hepatol Rep 3:1. doi:10.4103/2348-3113.141570
Barie PS, Eachempati SR (2010) Acute acalculous cholecystitis. Gastroenterol Clin N Am 39:343–357. doi:10.1016/j.gtc.2010.02.012, x
Karkera PJ, Sandlas G, Ranjan R, Gupta A, Kothari P (2010) Acute acalculous cholecystitis causing gall bladder perforation in children. J Indian Assoc Pediatr Surg 15:139–141. doi:10.4103/0971-9261.72439
Howard RJ (1981) Acute acalculous cholecystitis. Am J Surg 141:194–198
Anderson JE, Inui T, Talamini MA, Chang DC (2014) Cholecystostomy offers no survival benefit in patients with acute acalculous cholecystitis and severe sepsis and shock. J Surg Res 190:517–521. doi:10.1016/j.jss.2014.02.043
Herlin P, Ericsson M, Holmin T, Jonsson PE (1982) Acute acalculous cholecystitis following trauma. Br J Surg 69:475–476
Munster AM, Brown JR (1967) Acalculous cholecystitis. Am J Surg 113:730–734
Buyukasik Y, Kosar A, Demiroglu H, Altinok G, Ozcebe OI, Dundar S (1998) Acalculous acute cholecystitis in leukemia. J Clin Gastroenterol 27:146–148
Ganpathi IS, Diddapur RK, Eugene H, Karim M (2007) Acute acalculous cholecystitis: challenging the myths. HPB (Oxford) 9:131–134. doi:10.1080/13651820701315307
Savoca PE, Longo WE, Zucker KA, McMillen MM, Modlin IM (1990) The increasing prevalence of acalculous cholecystitis in outpatients. Results of a 7-year study. Ann Surg 211:433–437
Ryu JK, Ryu KH, Kim KH (2003) Clinical features of acute acalculous cholecystitis. J Clin Gastroenterol 36:166–169
Glenn F, Becker CG (1982) Acute acalculous cholecystitis. An increasing entity. Ann Surg 195:131–136
Csikesz NG, Tseng JF, Shah SA (2008) Trends in surgical management for acute cholecystitis. Surgery 144:283–289. doi:10.1016/j.surg.2008.03.033
Borzellino G, Sauerland S, Minicozzi AM, Verlato G, Di Pietrantonj C, de Manzoni G et al (2008) Laparoscopic cholecystectomy for severe acute cholecystitis. A meta-analysis of results. Surg Endosc 22:8–15. doi:10.1007/s00464-007-9511-6
Melloul E, Denys A, Demartines N, Calmes JM, Schafer M (2011) Percutaneous drainage versus emergency cholecystectomy for the treatment of acute cholecystitis in critically ill patients: does it matter? World J Surg 35:826–833. doi:10.1007/s00268-011-0985-y
Winbladh A, Gullstrand P, Svanvik J, Sandström P (2009) Systematic review of cholecystostomy as a treatment option in acute cholecystitis. HPB 11:183–193. doi:10.1111/j.1477-2574.2009.00052.x
Glenn F (1979) Acute acalculous cholecystitis. Ann Surg 189:458–465
Owen CC, Jain R (2005) Acute acalculous cholecystitis. Curr Treat Options Gastroenterol 8:99–104
Laurila J, Laurila PA, Saarnio J, Koivukangas V, Syrjala H, Ala-Kokko TI (2006) Organ system dysfunction following open cholecystectomy for acute acalculous cholecystitis in critically ill patients. Acta Anaesthesiol Scand 50:173–179
Tsakayannis DE, Kozakewich HPW, Lillehei CW (1996) Acalculous cholecystitis in children. J Pediatr Surg 31:127–131. doi:10.1016/S0022-3468(96)90334-6
Ferrarese F, Cecere V, Fabiano G (2006) Acute acalculous cholecystitis: pathophysiology and treatment. Ann Ital Chir 77:309–311
Kuster GG, Domagk D (1996) Laparoscopic cholecystostomy with delayed cholecystectomy as an alternative to conversion to open procedure. Surg Endosc 10:426–428
Bradley KM, Dempsey DT (2002) Laparoscopic tube cholecystostomy: still useful in the management of complicated acute cholecystitis. J Laparoendosc Adv Surg Tech A 12:187–191. doi:10.1089/10926420260188083
Koebrugge B, van Leuken M, Ernst MF, van Munster I, Bosscha K (2010) Percutaneous cholecystostomy in critically ill patients with a cholecystitis: a safe option. Dig Surg 27:417–421. doi:10.1159/000308460
Simorov A, Ranade A, Parcells J, Shaligram A, Shostrom V, Boilesen E et al (2013) Emergent cholecystostomy is superior to open cholecystectomy in extremely ill patients with acalculous cholecystitis: a large multicenter outcome study. Am J Surg 206:935–940. doi:10.1016/j.amjsurg.2013.08.019, discussion 940-1
Morse BC, Smith JB, Lawdahl RB, Roettger RH (2010) Management of acute cholecystitis in critically ill patients: contemporary role for cholecystostomy and subsequent cholecystectomy. Am Surg 76:708–712
Cha BH, Song HH, Kim YN, Jeon WJ, Lee SJ, Kim JD et al (2014) Percutaneous cholecystostomy is appropriate as definitive treatment for acute cholecystitis in critically ill patients: a single center, cross-sectional study. Korean J Gastroenterol 63:32–38
Granlund A, Karlson BM, Elvin A, Rasmussen I (2001) Ultrasound-guided percutaneous cholecystostomy in high-risk surgical patients. Langenbeck’s Arch Surg 386:212–217
Chung YH, Choi ER, Kim KM, Kim MJ, Lee JK, Lee KT et al (2012) Can percutaneous cholecystostomy be a definitive management for acute acalculous cholecystitis? J Clin Gastroenterol 46:216–219. doi:10.1097/MCG.0b013e3182274375
McClain T, Gilmore BT, Peetz M (1997) Laparoscopic cholecystectomy in the treatment of acalculus cholecystitis in patients after thermal injury. J Burn Care Rehabil 18:141–146
Soffer D, Blackbourne LH, Schulman CI, Goldman M, Habib F, Benjamin R et al (2007) Is there an optimal time for laparoscopic cholecystectomy in acute cholecystitis? Surg Endosc 21:805–809. doi:10.1007/s00464-006-9019-5
Soper NJ, Stockmann PT, Dunnegan DL, Ashley SW (1992) Laparoscopic cholecystectomy. The new ‘gold standard’? Arch Surg 127:917–921, discussion 921-3
Wiesen SM, Unger SW, Barkin JS, Edelman DS, Scott JS, Unger HM (1993) Laparoscopic cholecystectomy: the procedure of choice for acute cholecystitis. Am J Gastroenterol 88:334–337
Lutsevich EV, Gribkov I, Save’lev EA, Urbanovich AS (1989) Acute acalculous cholecystitis in emergency surgery. Khirurgiia (Mosk) 7:3–8
Almeida J, Sleeman D, Sosa JL, Puente I, McKenney M, Martin L (1995) Acalculous cholecystitis: the use of diagnostic laparoscopy. J Laparoendosc Surg 5:227–231
Arnot RS (1994) Laparoscopy and acalculous cholecystitis. Aust N Z J Surg 64:405–406
Dwivedi A, Shetty A, Sanghavi P, Phan T, Lakra Y, Silva Y (2004) Efficacy of laparoscopic cholecystectomy in acalculous gallbladder disease: long-term follow-up. JSLS 8:119–122
Frassinelli P, Werner M, Reed JF 3rd, Scagliotti C (1998) Laparoscopic cholecystectomy alleviates pain in patients with acalculous biliary disease. Surg Laparosc Endosc 8:30–34
Alimoglu O, Ozkan OV, Sahin M, Akcakaya A, Eryilmaz R, Bas G (2003) Timing of cholecystectomy for acute biliary pancreatitis: outcomes of cholecystectomy on first admission and after recurrent biliary pancreatitis. World J Surg 27:256–259. doi:10.1007/s00268-002-6647-3
Davis CA, Landercasper J, Gundersen LH, Lambert PJ (1999) Effective use of percutaneous cholecystostomy in high-risk surgical patients: techniques, tube management, and results. Arch Surg 134:727–731, discussion 731-2
Rattner DW, Ferguson C, Warshaw AL (1993) Factors associated with successful laparoscopic cholecystectomy for acute cholecystitis. Ann Surg 217:233–236
Peng WK, Sheikh Z, Paterson-Brown S, Nixon SJ (2005) Role of liver function tests in predicting common bile duct stones in acute calculous cholecystitis. Br J Surg 92:1241–1247. doi:10.1002/bjs.4955
Nasim S, Khan S, Alvi R, Chaudhary M (2011) Emerging indications for percutaneous cholecystostomy for the management of acute cholecystitis—a retrospective review. Int J Surg 9:456–459. doi:10.1016/j.ijsu.2011.04.008
Eggermont AM, Lameris JS, Jeekel J (1985) Ultrasound-guided percutaneous transhepatic cholecystostomy for acute acalculous cholecystitis. Arch Surg 120:1354–1356
Vauthey JN, Lerut J, Martini M, Becker C, Gertsch P, Blumgart LH (1993) Indications and limitations of percutaneous cholecystostomy for acute cholecystitis. Surg Gynecol Obstet 176:49–54
Chang YR, Ahn YJ, Jang JY, Kang MJ, Kwon W, Jung WH et al (2014) Percutaneous cholecystostomy for acute cholecystitis in patients with high comorbidity and re-evaluation of treatment efficacy. Surgery 155:615–622. doi:10.1016/j.surg.2013.12.026
Little MW, Briggs JH, Tapping CR, Bratby MJ, Anthony S, Phillips-Hughes J et al (2013) Percutaneous cholecystostomy: the radiologist’s role in treating acute cholecystitis. Clin Radiol 68:654–660. doi:10.1016/j.crad.2013.01.017
Griniatsos J, Petrou A, Pappas P, Revenas K, Karavokyros I, Michail OP et al (2008) Percutaneous cholecystostomy without interval cholecystectomy as definitive treatment of acute cholecystitis in elderly and critically ill patients. South Med J 101:586–590. doi:10.1097/SMJ.0b013e3181757b77
Berber E, Engle KL, String A, Garland AM, Chang G, Macho J et al (2000) Selective use of tube cholecystostomy with interval laparoscopic cholecystectomy in acute cholecystitis. Arch Surg 135:341–346
Ito K, Fujita N, Noda Y, Kobayashi G, Kimura K, Sugawara T et al (2004) Percutaneous cholecystostomy versus gallbladder aspiration for acute cholecystitis: a prospective randomized controlled trial. AJR Am J Roentgenol 183:193–196. doi:10.2214/ajr.183.1.1830193
Kim KH, Sung CK, Park BK, Kim WK, Oh CW, Kim KS (2000) Percutaneous gallbladder drainage for delayed laparoscopic cholecystectomy in patients with acute cholecystitis. Am J Surg 179:111–113. doi:10.1016/S0002-9610(00)00247-6
Boland GW, Lee MJ, Leung J, Mueller PR (1994) Percutaneous cholecystostomy in critically ill patients: early response and final outcome in 82 patients. AJR Am J Roentgenol 163:339–342. doi:10.2214/ajr.163.2.8037026
Shirai Y, Tsukada K, Kawaguchi H, Ohtani T, Muto T, Hatakeyama K (1993) Percutaneous transhepatic cholecystostomy for acute acalculous cholecystitis. Br J Surg 80:1440–1442
Lo LD, Vogelzang RL, Braun MA, Nemcek AA (1995) Percutaneous cholecystostomy for the diagnosis and treatment of acute calculous and acalculous cholecystitis. J Vasc Interv Radiol 6:629–634. doi:10.1016/S1051-0443(95)71150-2
Akhan O, Akinci D, Özmen MN (2002) Percutaneous cholecystostomy. Eur J Radiol 43:229–236. doi:10.1016/S0720-048X(02)00158-4
Atar E, Bachar GN, Berlin S, Neiman C, Bleich-Belenky E, Litvin S et al (2014) Percutaneous cholecystostomy in critically ill patients with acute cholecystitis: complications and late outcome. Clin Radiol 69:e247–e252. doi:10.1016/j.crad.2014.01.012
Zerem E, Omerovic S (2012) Can percutaneous cholecystostomy be a definitive management for both acute calculous and acalculous cholecystitis? J Clin Gastroenterol 46:251. doi:10.1097/MCG.0b013e3182333834
Dabus Gde C, Dertkigil SS, Baracat J (2003) Percutaneous cholecystostomy: a nonsurgical therapeutic option for acute cholecystitis in high-risk and critically ill patients. Sao Paulo Med J 121:260–262
Welschbillig-Meunier K, Pessaux P, Lebigot J, Lermite E, Aube C, Brehant O et al (2005) Percutaneous cholecystostomy for high-risk patients with acute cholecystitis. Surg Endosc 19:1256–1259. doi:10.1007/s00464-004-2248-6
Anderson JE, Chang DC, Talamini MA (2013; 2013) A nationwide examination of outcomes of percutaneous cholecystostomy compared with cholecystectomy for acute cholecystitis, 1998–2010. Surg Endosc 27:3406–3411. doi:10.1007/s00464-013-2924-5
Nguyen NT, Hinojosa M, Fayad C, Varela E, Wilson SE (2007) Use and outcomes of laparoscopic versus open gastric bypass at academic medical centers. J Am Coll Surg 205:248–255
Hinojosa MW, Murrell ZA, Konyalian VR, Mills S, Nguyen NT, Stamos MJ (2007) Comparison of laparoscopic vs open sigmoid colectomy for benign and malignant disease at academic medical centers. J Gastrointest Surg 11:1423–1429. doi:10.1007/s11605-007-0269-x, discussion 1429-30
Acknowledgments
The authors acknowledge support from the following source: The Center for Advanced Surgical Technology at the University of Nebraska Medical Center.
Authors’ contributions
Dmitry Oleynikov, MD did the study conception and design. Charles Treinen, BS and Daniel Lomelin, MPH performed the acquisition of data and did the analysis and interpretation of data. Charles Treinen, BS; Daniel Lomelin, MPH; and Crystal Krause, PhD drafted the manuscript. Charles Treinen, BS; Daniel Lomelin, MPH; Crystal Krause, PhD; and Dmitry Oleynikov, MD critically revised the manuscript.
Ethics statement
This study utilized the University HealthSystem Consortium database, which uses de-identified patient information, and as such does not require informed consent or IRB approval. The manuscript does not contain clinical studies or individual patient data.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Treinen, C., Lomelin, D., Krause, C. et al. Acute acalculous cholecystitis in the critically ill: risk factors and surgical strategies. Langenbecks Arch Surg 400, 421–427 (2015). https://doi.org/10.1007/s00423-014-1267-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-014-1267-6