Abstract
Background
The current study aims to investigate the impact of baseline characteristics on the outcomes of sorafenib-treated advanced Hepatocellular carcinoma (HCC) patients in the setting of a clinical trial.
Methods
This is a secondary analysis of the comparator arm (sorafenib arm) of the NCT00699374 study which is a phase III multicenter study conducted between 2008 and 2010. The univariate probability of overall and progression-free survival was assessed among different patient subsets through Kaplan–Meier analysis and log-rank testing. Multivariate analysis of factors affecting overall and progression-free survival was then conducted through Cox regression analysis.
Results
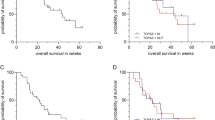
All patients within the comparator (sorafenib) arm were included in the analysis (N = 544 patients). In multivariate analysis, prior hepatectomy (P = 0.028), prior locoregional treatment (P = 0.048), grade 1 ALBI score (P < 0.001), ECOG performance score of 0 (P < 0.001), BMI ≥ 25 (P = 0.026), AFP < 200 (P = 0.001), and no extra-hepatic spread (P = 0.007) were associated with better overall survival. Likewise, in multivariate analysis, non-Asian race (P = 0.004), grade 1 ALBI score (P = 0.001), ECOG performance score of 0 (P = 0.006), and no extra-hepatic spread (P = 0.005) were associated with better progression-free survival. Moreover, development of high-grade hand–foot skin reaction was associated with a statistically significant improvement in overall survival (P = 0.003), which was further confirmed in a multivariate analysis adjusted for other relevant baseline factors (P = 0.002).
Conclusion
Within a cohort of highly selected advanced HCC patients, baseline patient-, liver-, and disease-centered variables play an important role in predicting patient outcomes. This information is important in terms of therapeutic decision-making and patient counseling.

Similar content being viewed by others
References
Abdel-Rahman O (2017a) Assessment of the discriminating value of the 8th AJCC stage grouping for hepatocellular carcinoma. HPB (Oxford)
Abdel-Rahman O (2017b) Role of liver-directed local tumor therapy in the management of hepatocellular carcinoma with extrahepatic metastases: a SEER database analysis. Expert Rev Gastroenterol Hepatol 11(2):183–189
Abdel-Rahman O, Elsayed Z (2017) External beam radiotherapy for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev (3)
Abdel-Rahman O, Fouad M (2014) Sorafenib-based combination as a first line treatment for advanced hepatocellular carcinoma: a systematic review of the literature. Crit Rev Oncol Hematol 91(1):1–8
Abdel-Rahman O, Lamarca A (2017) Development of sorafenib-related side effects in patients diagnosed with advanced hepatocellular carcinoma treated with sorafenib: a systematic-review and meta-analysis of the impact on survival. Expert Rev Gastroenterol Hepatol 11(1):75–83
Bruix J, Cheng A, Kang Y, Tsao C, Qin S, Lentini G et al (2009) Effect of macroscopic vascular invasion (MVI), extrahepatic spread (EHS), and ECOG performance status (ECOG PS) on outcome in patients with advanced hepatocellular carcinoma (HCC) treated with sorafenib: Analysis of two phase III, randomized, double-blind trials. J Clin Oncol 27(15S):4580-
Cheng A-L, Kang Y-K, Chen Z, Tsao C-J, Qin S, Kim JS et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10(1):25–34
Choo SP, Tan WL, Goh BKP, Tai WM, Zhu AX (2016) Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer 122(22):3430–3446
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359-86
Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL et al (2015) Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach—the ALBI grade. J Clin Oncol 33(6):550–558
Lamarca A, Abdel-Rahman O, Salu I, McNamara MG, Valle JW, Hubner RA (2016) Identification of clinical biomarkers for patients with advanced hepatocellular carcinoma receiving sorafenib. Clin Transl Oncol
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390
Lue A, Serrano MT, Bustamante FJ, Inarrairaegui M, Arenas JI, Testillano M et al (2017) Neutrophil-to-lymphocyte ratio predicts survival in European patients with hepatocellular carcinoma administered sorafenib. Oncotarget 8(61):103077–103086
Marrero JA, Kudo M, Venook AP, Ye SL, Bronowicki JP, Chen XP et al (2016) Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J Hepatol 65(6):1140–1147
Mir O, Coriat R, Blanchet B, Durand J-P, Boudou-Rouquette P, Michels J et al (2012) Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS One 7(5):e37563
Mittal S, El-Serag HB (2013) Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 47(Suppl):S2–S6
Nada Y, Rashad N, Eissa M, Ghonaim A, Farag K, Saadawi I et al (2017) Outcomes of treatment with sorafenib in Egyptian patients with hepatocellular carcinoma: a retrospective cohort study. Expert Rev Gastroenterol Hepatol 1–9
Nishikawa H, Nishijima N, Enomoto H, Sakamoto A, Nasu A, Komekado H et al (2017) Predictive factors in patients with hepatocellular carcinoma receiving sorafenib therapy using time-dependent receiver operating characteristic analysis. J Cancer 8(3):378–387
Oweira H, Helbling D, Petrausch U et al (2017) Early stage Hepatocellular carcinoma in the elderly: a SEER database analysis. J Geriatric Oncol (in press)
Parikh ND, Marshall VD, Singal AG, Nathan H, Lok AS, Balkrishnan R et al (2017) Survival and cost-effectiveness of sorafenib therapy in advanced hepatocellular carcinoma: an analysis of the SEER-Medicare database. Hepatology 65(1):122–133
Qin S, Yang T, Tak W, Yu S, Tsao C, Kim J et al (2009) Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma (HCC): Asia-Pacific (AP) trial subgroup analyses by baseline transaminase (ALT/AST)/α-fetoprotein (AFP) levels. J Clin Oncol 27(15S):4590-
Raoul J, Craxi A, Porta C, Lentini G, Nadel A, Voliotis D et al (2009) Impact of lymph node metastases on outcome following treatment with sorafenib in patients with hepatocellular carcinoma (HCC): subset analysis from the phase III SHARP trial. J Clin Oncol 27(15S):e15547
Yang T, Qin S, Tak W, Yu S, Tsao C, Kim J et al (2009) Impact of prior surgical resection with curative intent on the efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma (HCC): subset analysis of the Asia-Pacific (AP) study. J Clin Oncol 27(15S):e15518
Acknowledgements
This publication is based on research using information obtained from http://www.projectdatasphere.org, which is maintained by Project Data Sphere, LLC. Neither Project Data Sphere, LLC, nor the owner(s) of any information from the web site have contributed to, approved or are in any way responsible for the contents of this publication.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author. The current study is fully compliant with all applicable ethical and legal standards. It complies fully with the Declaration of Helsinki.
Informed consent
As this study is based on a publicly available data set without identifying patient information, informed consent was not needed.
Rights and permissions
About this article
Cite this article
Abdel-Rahman, O. Impact of baseline characteristics on outcomes of advanced HCC patients treated with sorafenib: a secondary analysis of a phase III study. J Cancer Res Clin Oncol 144, 901–908 (2018). https://doi.org/10.1007/s00432-018-2610-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2610-z