Abstract
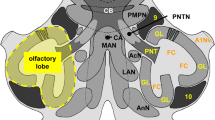
The nervus terminalis (NT) is the most anterior of the vertebrate cranial nerves. In teleost fish, the NT runs across all olfactory components and shows high morphological variability within this taxon. We compare the anatomical distribution, average number and size of the FMRFamide-immunoreactive (ir) NT cells of fourteen teleost species with different positions of olfactory bulbs (OBs) with respect to the ventral telencephalic area. Based on the topology of the OBs, three different neuroanatomical organizations of the telencephalon can be defined, viz., fish having sessile (Type I), pseudosessile (short stalked; Type II) or stalked (Type III) OBs. Type III topology of OBs appears to be a feature associated with more basal species, whereas Types I and II occur in derived and in basal species. The displacement of the OBs is positively correlated with the peripheral distribution of the FMRFamide-ir NT cells. The number of cells is negatively correlated with the size of the cells. A dependence analysis related to the type of OB topology revealed a positive relationship with the number of cells and with the size of the cells, with Type I and II topologies of OBs showing significantly fewer cells and larger cells than Type III. A dendrogram based on similarities obtained by taking into account all variables under study, i.e., the number and size of the FMRFamide-ir NT cells and the topology of OBs, does not agree with the phylogenetic relationships amongst species, suggesting that divergent or convergent evolutionary phenomena produced the olfactory components studied.





Similar content being viewed by others
References
Barton RA, Harvey PH (2000) Mosaic evolution of brain structures in mammals. Nature 405:1055–1058
Batten TFC, Cambre ML, Moons L, Vandesande F (1990) Comparative distribution of neuropeptide-immunoreactive systems in the brain of the green molly, Poecilia latipinna. J Comp Neurol 302:893–919
Betancur-R R, Broughton RE, Wiley EO, Carpenter K, López JA, Li C, Holcroft NI, Arcila D, Sanciangco M, Cureton Ii JC, Zhang F, Buser T, Campbell MA, Ballesteros JA, Roa-Varon A, Willis S, Borden WC, Rowley T, Reneau PC, Hough DJ, Lu G, Grande T, Arratia G, Ortí G (2013) The tree of life and a new classification of bony fishes. PLoS Curr 18:5
Biju KC, Singru PS, Schreibman MP, Subhedar N (2003) Reproduction phase-related expression of GnRH-like immunoreactivity in the olfactory receptor neurons, their projections to the olfactory bulb and in the nervus terminalis in the female Indian major carp Cirrhinus mrigala (Ham.). Gen Comp Endocrinol 133:358–367
Bonn U, König B (1988) FMRFamide-like immunoreactivity in Brain and pituitary of Xenotoca-eisenii: (Cyprinidontoformes, Teleostei). J Hirnforsch 29:121–131
Bonn U, König B (1989a) FMRFamide immunoreactivity in the brain and pituitary of Carassius auratus (Cyprinidae, Teleostei). J Hirnforsch 30:361–370
Bonn U, König B (1989b) FMRFamide-like immunoreactivity in the brain and pituitary of the teleost—Eigenmannia lineata (Gymnotiformes). Z Mikrosk Anat Forsch 103:221–236
Brookover C, Jackson TS (1911) The olfactory nerve and the nervus terminalis of Ameiurus. J Comp Neurol 21:237–259
Castro A, Becerra M, Anadón R, Manso MJ (2001) Distribution and development of FMRFamide-like immunoreactive neuronal systems in the brain of the brown trout, Salmo trutta fario. J Comp Neurol 440:43–64
Chiba A (1997) Co-localization of gonadotropin-releasing hormone (GnRH)-, neuropeptide Y (NPY)-, and molluscan cardioexcitatory tetrapeptide (FMRFamide)-like immunoreactivities in the ganglion cells of the terminal nerve of the masu salmon. Fish Sci 63:153–154
Chiba A, Sohn YC, Honma Y (1996) Immunohistochemical and ultrastructural characterization of the terminal nerve ganglion cells of the ayu, Plecoglossus altivelis (Salmoniformes, Teleostei). Anat Rec 246:549–556
D’Aniello B, Luongo L, Rastogi RK, di Meglio M, Pinelli C (2015) Tract-tracing study of the extrabulbar olfactory projections in the brain of some teleosts. Microsc Res Tech 78:268–276
de Winter W, Oxnard CE (2001) Evolutionary radiations and convergences in the structural organization of mammalian brains. Nature 409:710–714
Eifert C, Farnworth M, Schulz-Mirbach T, Riesch R, Bierbach D, Klaus S, Wurster A, Tobler M, Streit B, Indy JR, Arias-Rodriguez L, Plath M (2014) Brain size variation in extremophile fish: local adaptation vs. phenotypic plasticity. J Zool 295:143–153
Eisthen HL, Delay RJ, Wirsig-Wiechmann CR, Dionne VE (2000) Neuromodulatory effects of gonadotropin releasing hormone on olfactory receptor neurons. J Neurosci 20:3947–3955
Ekström P, Honkanen T, Ebbesson SO (1988) FMRFamide-like immunoreactive neurons of the nervus terminalis of teleosts innervate both retina and pineal organ. Brain Res 460:68–75
Finlay BL, Darlington RB (1995) Linked regularities in the development and evolution of mammalian brains. Science 268:1578–1584
Finlay BL, Darlington RB, Nicastro N (2001) Developmental structure in brain evolution. Behav Brain Sci 24:298–308
Fiorentino M, D’Aniello B, Joss J, Polese G, Rastogi RK (2002) Ontogenetic organization of the FMRFamide immunoreactivity in the nervus terminalis of the lungfish, Neoceratodus forsteri. J Comp Neurol 450:115–121
Fritsch G (1878) Untersuchungen über den feineren Bau des Fischgehirns mit besonderer Berücksichtigung der Homologien bei anderen Wirbelthierklassen. Verlag der Gutmann’schen Buchhandlung, Berlin
Fujii K, Kobayashi H (1992) FMRFamide-like immunoreactivity in the brain and pituitary of the goldfish, Carassius auratus. Ann Anat 174:217–222
Gonda A, Herczeg G, Merilä J (2009) Adaptative brain size divergence in nine-spined sticklebacks (Pungitius pungitius)? J Evol Biol 22:1721–1726
Gonda A, Herczeg G, Merilä J (2011) Population variation in brain size of nine-spined sticklebacks (Pungitius pungitius)—local adaptation or environmentally induced variation? BMC Evol Biol 11:75–77
Gonda A, Välimäki K, Herczeg G, Merilä J (2012) Brain development and predation: plastic responses depend on evolutionary history. Biol Lett 8:249–252
Gonda A, Herczeg G, Merilä J (2013) Evolutionary ecology of intraspecific brain size variation: a review. Ecol Evol 3:2751–2764
Gonzalez-Voyer A, Winberg S, Kolm N (2009) Brain structure evolution in a basal vertebrate clade: evidence from phylogenetic comparative analysis of cichlid fishes. BMC Evol Biol 9:238
Gonzalez-Voyer A, Kolm N, Iwaniuk A (2010) Sex, ecology and the brain: evolutionary correlates of brain structure volumes in Tanganyikan cichlids. PLoS ONE 5:e14355
Hager R, Lu L, Rosen GD, Williams RW (2012) Genetic architecture supports mosaic brain evolution and independent brain-body size regulation. Nat Commun 3:1079
Holmgren N, van der Horst CJ (1925) Contribution to the morphology of the brain of Ceratodus. Acta Zool 6:59–165
Huber R, van Staaden MJ, Kaufman LS, Liem KF (1997) Microhabitat use, trophic patterns, and the evolution of brain structure in African cichlids. Brain Behav Evol 50:167–182
Jadhao AG, D’Aniello B, Malz CR, Pinelli C, Meyer DL (2001) Intrasexual and intersexual dimorphisms of the red salmon prosencephalon. Cell Tissue Res 304:121–140
Kawai T, Oka Y, Eisthen H (2009) The role of the terminal nerve and GnRH in olfactory system neuromodulation. Zool Sci 26:669–680
Kihslinger RL, Lema SC, Nevitt GA (2006) Environmental rearing conditions produce forebrain differences in wild Chinook salmon Oncorhynchus tshawytscha. Comp Biochem Physiol 145:145–151
Kim MH, Oka Y, Amano M, Kobayashi M, Okuzawa K, Hasegawa Y, Kawashima S, Suzuki Y, Aida K (1995) Immunocytochemical localization of sGnRH and cGnRH-II in the brain of goldfish, Carassius auratus. J Comp Neurol 356:72–82
Kotrschal K, van Staaden M, Huber R (1998) Fish brains: evolution and environmental relationships. Rev Fish Biol Fish 8:373–408
Kotrschal A, Rogell B, Maklakov AA, Kolm N (2012) Sex-specific plasticity in brain morphology depends on social environment of the guppy, Poecilia reticulata. Behav Ecol Sociobiol 66:1485–1492
Kyle AL, Luo BG, Magnus TH, Stell WK (1995) Substance P-, F8Famide-, and A18Famide-like immunoreactivity in the nervus terminalis and retina of the goldfish Carassius auratus. Cell Tissue Res 280:605–615
Lecchini D, Lecellier G, Lanyon RG, Holles S, Poucet B, Duran E (2014) Variation in brain organization of coral reef fish larvae according to life history traits. Brain Behav Evol 83:17–30
Lema SC, Hodges MJ, Marchetti MP, Nevitt GA (2005) Proliferation zones in the salmon telencephalon and evidence for environmental influence on proliferation rate. Comp Biochem Physiol 141:327–335
Magliulo-Cepriano L, Schreibman MP, Blüm V (1993) The distribution of immunoreactive FMRF-amide, neurotensin, and galanin in the brain and pituitary gland of three species of Xiphophorus from birth to sexual maturity.Gen Comp Endocrinol 92:269–280
Mathieu M, Tagliafierro G, Bruzzone F, Vallarino M (2002) Neuropeptide tyrosine-like immunoreactive system in the brain, olfactory organ and retina of the zebrafish, Danio rerio, during development. Brain Res Dev Brain Res 139:255–265
Münz H, Claas B (1987) The terminal nerve and its development in teleost fishes. In: Demski LS, Schwanzel-Fukuda M (eds) The terminal nerve (nervus terminalis): structure, function, and evolution. New York Academy of Sciences, New York, pp 50–59
Münz H, Stumpf WE, Jennes L (1981) LHRH systems in the brain of platyfish. Brain Res 221:1–13
Nieuwenhuys R (1967) Comparative anatomy of the olfactory centers and tracts. Prog Brain Res 23:1–64
Nieuwenhuys R, Donkelaar HJT, Nicholson C (1998) The central nervous system of vertebrates. Springer, Heidelberg
Ogawa S, Akiyama G, Kato S, Soga T, Sakuma Y, Parhar IS (2006) Immunoneutralization of gonadotropin-releasing hormone type-III suppresses male reproductive behavior of cichlids. Neurosci Lett 403:201–205
Oka Y, Ichikawa M (1990) Gonadotropin-releasing hormone (GnRH) immunoreactive system in the brain of the dwarf gourami (Colisa lalia) as revealed by light microscopic immunocytochemistry using a monoclonal antibody to common amino acid sequence of GnRH. J Comp Neurol 300:511–522
Oka Y, Munro AD, Lam TJ (1986) Retinopetal projections from a subpopulation of ganglion cells of the nervus terminalis in the dwarf gourami (Colisa lalia). Brain Res 367:341–345
Okuyama T, Yokoi S, Abel H, Isoe Y, Suehiro Y, Imada H, Tanaka M, Kawasaki T, Yuba S, Taniguchi Y, Kamei Y, Okubo K, Shimada A, Naruse K, Takeda H, Oka Y, Kubo T, Takeuchi H (2014) A neural mechanism underlying mating preferences for familiar individuals in Medaka fish. Science 343:91–94
Östholm T, Ekström P, Ebbesson SOE (1989) FMRFamide-like immunoreactive neurons in presmolt, postsmolt and adult coho salmon (Oncorhynchus kisutch). Anat Rec 223:86A
Östholm T, Ekström P, Ebbesson SOE (1990) Distribution of FMRFamide-like immunoreactivity in the brain, retina and nervus terminalis of the sockeye salmon parr, Oncorhynchus nerka. Cell Tissue Res 261:403–418
Parhar IS (2002) Cell migration and evolutionary significance of GnRH subtypes. Prog Brain Res 141:3–17
Parhar IS, Pfaff DW, Schwanzel-Fukuda M (1996) Gonadotropin-releasing hormone gene expression in teleosts. Mol Brain Res 41:216–227
Pinelli C, D’Aniello B, Sordino P, Meyer DL, Fiorentino M, Rastogi RK (2000) Comparative immunocytochemical study of FMRFamide neuronal system in the brain of Danio rerio and Acipenser ruthenus during development. Dev Brain Res 119:195–208
Pinelli C, Rastogi RK, Scandurra A, Jadhao AG, Aria M, D’Aniello B (2014) A comparative cluster analysis of adenine dinucleotide phosphate (NADPH)-diaphorase histochemistry in the brains of amphibians. J Comp Neurol 522:2980–3003
Pinkus F (1894) Über einen noch nicht beschriebenen Hirnnerven des Protopterus annectens. Anat Anz 9:562–566
Pinkus F (1895) Die Hirnnerven des Protopterus annectens. Morph Arb 4:275–346
Pollen AA, Dobberfuhl AP, Scace J, Igulu MM, Renn SC, Shumway CA, Hofmann HA (2007) Environmental complexity and social organization sculpt the brain in Lake Tanganyikan cichlid fish. Brain Behav Evol 70:21–39
Rama Krishna NS, Subhedar NK (1992) Distribution of FMRFamide-like immunoreactivity in the forebrain of the catfish, Clarias batrachus (Linn.). Peptides 13:183–191
Ridet JM, Bauchot R (1990) Analyse quantitative de l’encéphale des Téléostéens, caractères évolutifs et adaptifs de l’encéphalisation. II. Les grandes subdivisions encéphaliques. J Hirnforsch 31:433–458
Rossi A, Basile A (1968) Comparative study of nervus terminalis ganglion cell topography in teleostei. Atti Accad Naz dei Lin 45:635
Rusoff AC, Hapner SJ (1990a)Organization of retinopetal axons in the optic nerve of the cichlid fish, Herotilapia multispinosa.J Comp Neurol 294:418–430
Rusoff AC, Hapner SJ (1990b) Development of retinopetal projections in the cichlid fish, Herotilapia multispinosa.J Comp Neurol 294:431–442
Salas C, Broglio C, Duran E, Gomez A, Rodriguez F (2008) Spatial learning in fish. In: Menzel R (ed) Learning theory and behavior. Elsevier, Oxford, pp 499–528
Schreibman MP, Halpern LR, Goos HJ, Margolis-Kazan H (1979) Identification of luteinizing hormone-releasing hormone (LH-RH) in the brain and the pituitary gland of a fish by immunocytochemistry. J Exp Zool 210:153–160
Sewertzoff AN (1902) Zur Entwicklungsgeschichte des Ceratodus forsteri. Anat Anz 21:593–608
Springer AD (1983) Centrifugal innervation of goldfish retina from ganglion cells of the nervus terminalis. J Comp Neurol 214:404–415
Stell WK, Walker SE, Chohan KS, Ball AK (1984) The goldfish nervus terminalis: a luteinizing hormone-releasing hormone and molluscan cardioexcitatory peptide immunoreactive olfactoretinal pathway. Proc Natl Acad Sci U S A 81:940–944
Stell WK, Walker SE, Ball AK (1987) Functional-anatomical studies on the terminal nerve projection to the retina of bony fishes. Ann NY Acad Sci 519:80–96
Striedter GF (2005) Principles of brain evolution. Sinauer, Sunderland
Szabo T, Blähser S, Denizot J-P, Ravaille-Véron M (1991) The olfactoretinalis system = terminal nerve? Neuroreport 2:73–76
Vecino E, Ekström P (1992) Colocalization of neuropeptide Y (NPY)-like and FMRFamide-like immunoreactivities in the brain of the Atlantic salmon (Salmo salar). Cell Tissue Res 270:435–442
von Bartheld CS, Meyer DL (1986) Tracing of single fibers of the nervus terminalis in the goldfish brain. Cell Tissue Res 245:143–158
von Bartheld CS, Meyer DL (1988) Central projections of the nervus terminalis in lampreys, lungfishes, and bichirs. Brain Behav Evol 32:151–159
Walker SE, Stell WK (1986) Gonadotropin-releasing hormone (GnRH), molluscan cardioexcitatory peptide (FMRFamide), enkephalin and related neuropeptides affect goldfish retinal ganglion cell activity. Brain Res 384:262–273
Whitlock KE (2004) Development of the nervus terminalis: origin and migration. Microsc Res Tech 65:2–12
Wirsig CR, Leonard CM (1987) Terminal nerve damage impairs the mating behavior of the male hamster. Brain Res 417:293–303
Wirsig-Wiechmann CR, Oka Y (2002) The terminal nerve ganglion cells project to the olfactory mucosa in the dwarf gourami. Neurosci Res 44:337–341
Yamamoto N, Oka Y, Amano M, Aida K, Hasegawa Y, Kawashima S (1995) Multiple gonadotropin-releasing hormone (GnRH)-immunoreactive systems in the brain of the dwarf gourami, Colisa lalia: immunohistochemistry and radioimmunoassay. J Comp Neurol 355:354–368
Yamamoto N, Oka Y, Kawashima S (1997) Lesions of gonadotropin-releasing hormone-immunoreactive terminal nerve cells: effects on the reproductive behavior of male dwarf gouramis. Neuroendocrinology 65:403–412
Yopak KE, Lisney TJ, Darlington RB, Collin SP, Montgomery JC, Finlay BL (2010) A conserved pattern of brain scaling from sharks to primates. Proc Natl Acad Sci U S A 107:12946–12951
Acknowledgments
The authors wish to thank Prof. William K. Stell for his valuable advice regarding the interpretation of the data.
Author information
Authors and Affiliations
Corresponding author
Additional information
The University of Naples “Federico II” (research grants from M.I.U.R.: PRIN 2011) and the Second University of Naples provided financial support.
The authors declare no identified conflicts of interest.
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The responsible authors were: for study concept and design, G.P., B.D., C.P.; for acquisition of data, G.P., L.L., A.S., L.M.; for analysis and interpretation of data, B.D., G.P. C.P.; for drafting of the manuscript, B.D., C.P.; for statistical analysis, M.A.
Rights and permissions
About this article
Cite this article
D’Aniello, B., Polese, G., Luongo, L. et al. Neuroanatomical relationships between FMRFamide-immunoreactive components of the nervus terminalis and the topology of olfactory bulbs in teleost fish. Cell Tissue Res 364, 43–57 (2016). https://doi.org/10.1007/s00441-015-2295-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2295-4