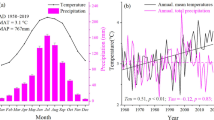
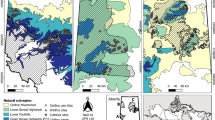
Abstract
Although negative density dependence (NDD) can facilitate tree species coexistence in forests, the underlying mechanisms can differ, and rarely are the dynamics of seedlings and saplings studied together. Herein we present and discuss a novel mechanism based on our investigation of NDD predictions for the large, grove-forming ectomycorrhizal mast fruiting tree, Microberlinia bisulcata (Caesalpiniaceae), in an 82.5-ha plot at Korup, Cameroon. We tested whether juvenile density, size, growth and survival decreases with increasing conspecific adult basal area for 3245 ‘new’ seedlings and 540 ‘old’ seedlings (< 75-cm tall) during an approximately 4-year study period (2008–2012) and for 234 ‘saplings’ (≥ 75-cm tall) during an approximately 6-year study period (2008–2014). We found that the respective densities of new seedlings, old seedlings and saplings were positively, not and negatively related to increasing BA. Maximum leaf numbers and heights of old seedlings were negatively correlated with increasing basal areas, as were sapling heights and stem diameters. Whereas survivorship of new seedlings decreased by more than one-half with increasing basal area over its range in 2010–2012, that of old seedlings decreased by almost two-thirds, but only in 2008–2010, and was generally unrelated to conspecific seedling density. In 2010–2012 relative growth rates in new seedlings’ heights decreased with increasing basal area, as well as with increasing seedling density, together with increasing leaf numbers, whereas old seedlings’ growth was unrelated to either conspecific density or basal area. Saplings of below-average height had reduced survivorship with increasing basal area (probability decreasing from approx. 0.4 to 0.05 over the basal area range tested), but only sapling growth in terms of leaf numbers decreased with increasing basal area. These static and dynamic results indicate that NDD is operating within this system, possibly stabilizing the M. bisulcata population. However, these NDD patterns are unlikely to be caused by symmetric competition or by consumers. Instead, an alternative mechanism for conspecific adult–juvenile negative feedback is proposed, one which involves the interaction between tree phenology and ectomycorrhizal linkages.





Similar content being viewed by others
References
Afshartous D, Preston RA (2011) Key results of interaction models with centering. J Stat Educ 19:1–23
Aiken LS, West SG (1991) Multiple regression: testing and interpreting interactions. SAGE Publications, London
Alexander IJ, Lee SS (2005) Mycorrhizas and ecosystem processes in tropical rain forest: implications for diversity. In: Burslem DRP, Pinard MA, Hartley SE (eds) Biotic interactions in the tropics. Cambridge University Press, Cambridge, pp 165–203
Alvarez-Buylla ER (1994) Density-dependence and patch dynamics in tropical rain-forests—matrix models and applications to a tree species. Am Nat 143:155–191
Aubréville A (1970) Légumineuses (Cesalpinioidees). Museum National d’Histoire Naturelle, Paris
Bagchi R, Henrys PA, Brown PE, Burslem DFRP, Diggle PJ, Gunatilleke CVS, Gunatilleke IAUN, Kassim AR, Law R, Noor S, Valencia RL (2011) Spatial patterns reveal negative density dependence and habitat associations in tropical trees. Ecology 92:1723–1729
Berryman AA (2002) Population: a central concept for ecology? Oikos 97:439–442
Blundell AG, Peart DR (2004) Density-dependent population dynamics of a dominant rain forest canopy tree. Ecology 85:704–715
Booth MG, Hoeksema JD (2010) Mycorrhizal networks counteract competitive effects of canopy trees on seedling survival. Ecology 91:2294–2302
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31:343–366
Chisholm RA, Condit R, Abd Rahman K, Baker PJ, Bunyavejchewin S, Chen YY, Chuyong G, Dattaraja HS, Davies S, Ewango CEN, Gunatilleke CVS, Gunatilleke IAUN, Hubbell S, Kenfack D, Kiratiprayoon S, Lin Y, Makana JR, Pongpattananurak N, Pulla S, Punchi-Manage R, Sukumar R, Su SH, Sun IF, Suresh HS, Tan S, Thomas D, Yap S (2014) Temporal variability of forest communities: empirical estimates of population change in 4000 tree species. Ecol Lett 17:855–865
Comita LS, Queenborough SA, Murphy SJ, Eck JL, Xu K, Krishnadas M, Beckman N, Zhu Y (2014) Testing predictions of the Janzen–Connell hypothesis: a meta-analysis of experimental evidence for distance- and density-dependent seed and seedling survival. J Ecol 102:845–856
Connell JH (1971) On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In: den Boer PJ, Gradwell GR (eds) Dynamics of populations. PUDOC, Wageningen, pp 298–312
Connell JH, Lowman DM (1989) Low-diversity tropical rain forests: some possible mechanisms for their existence. Am Nat 134:88–119
Denslow JS (1987) Tropical rain-forest gaps and tree species-diversity. Annu Rev Ecol Syst 18:431–451
Frazer GW, Canham CD, Lertzman KP (1999) Gap light analyzer (GLA), Version 2.0: Imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs. Simon Fraser University, Burnaby, BC, Canada, and the Institute of Ecosystem Studies, Millbrook, New York
Galwey NW (2006) Introduction to mixed modelling: beyond regression and analysis of variance. Wiley, West Sussex
Green JJ, Newbery DM (2001a) Light and seed size affect establishment of grove-forming ectomycorrhizal rain forest tree species. New Phytol 151:271–289
Green JJ, Newbery DM (2001b) Shade and leaf loss affect establishment of grove-forming rain forest tree species. New Phytol 151:291–309
Green JJ, Newbery DM (2002) Reproductive investment and seedling survival of the mast-fruiting rain forest tree, Microberlinia bisulcata A. chev. Plant Ecol 162:169–183
Harper JL (1977) Population biology of plants. Academic Press, London
Herrando-Perez S, Delean S, Brook BW, Bradshaw CJA (2012) Density dependence: an ecological Tower of Babel. Oecologia 170:585–603
Hixon MA, Pacala SW, Sandin SA (2002) Population regulation: historical context and contemporary challenges of open vs. closed systems. Ecology 83:1490–1508
Janzen DH (1970) Herbivores and number of tree species in tropical forests. Am Nat 104:501–528
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol 135:575–586
Johnson DJ, Beaulieu WT, Bever JT, Clay K (2012) Conspecific negative density dependence and forest diversity. Science 336:904–907
Jones FA, Comita LS (2010) Density-dependent pre-dispersal seed predation and fruit set in a tropical tree. Oikos 119:1841–1847
Kelly D, Sork VL (2002) Mast seeding in perennial plants: Why, how, where? Annu Rev Ecol Syst 33:427–447
Koide RT, Dickie IA (2002) Effects of mycorrhizal fungi on plant populations. Plant Soil 244:307–317
Krebs CJ (2002) Two complementary paradigms for analysing population dynamics. Philos Trans R Soc B 357:1211–1219
Lan G, Zhu H, Cao M, Hu Y, Wang H, Deng X, Zhou S, Cui J, Huang J, He Y, Liu L, Xu H, Song J (2009) Spatial dispersion patterns of trees in a tropical rainforest in Xishuangbanna, southwest China. Ecol Res 24:1117–1124
Letouzey R (1968) Etude phytogéographique du Cameroun. P. LeChevalier, Paris
Mangan SA, Schnitzer SA, Herre EA, Mack KML, Valencia MC, Sanchez EI, Bever JD (2010) Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature 466:752–755
Maron JL, Crone E (2006) Herbivory: effects on plant abundance, distribution and population growth. Proc R Soc Biol Sci 273:2575–2584
Matos DMS, Freckleton RP, Watkinson AR (1999) The role of density dependence in the population dynamics of a tropical palm. Ecology 80:2635–2650
McCarthy-Neumann S, Kobe RK (2010) Conspecific plant–soil feedbacks reduce survivorship and growth of tropical tree seedlings. J Ecol 98:396–407
McGuire KL (2007) Common ectomycorrhizal networks may maintain monodominance in a tropical rain forest. Ecology 88:567–574
Newbery DM, Gartlan JS (1996) A structural analysis of rain forest at Korup and Doula-Edea, Cameroon. Proc R Soc Edinb B 104:177–224
Newbery DM, Stoll P (2013) Relaxation of species-specific neighborhood effects in Bornean rain forest under climatic perturbation. Ecology 94:2838–2851
Newbery DM, Alexander IJ, Thomas DW, Gartlan JS (1988) Ectomycorrhizal rain-forest legumes and soil-phosphorus in Korup National Park, Cameroon. New Phytol 109:433–450
Newbery DM, Alexander IJ, Rother JA (1997) Phosphorus dynamics in a lowland African rain forest: the influence of ectomycorrhizal trees. Ecol Monogr 67:367–409
Newbery DM, Songwe NC, Chuyong GB (1998) Phenology and dynamics of an African rainforest at Korup, Cameroon. In: Newbery DM, Prins HHT, Brown ND (eds) Dynamics of tropical communities. Blackwell Science, Oxford, pp 267–308
Newbery DM, Alexander IJ, Rother JA (2000) Does proximity to conspecific adults influence the establishment of ectomycorrhizal trees in rain forest? New Phytol 147:401–409
Newbery DM, van der Burgt XM, Moravie MA (2004) Structure and inferred dynamics of a large grove of Microberlinia bisulcata trees in central African rain forest: the possible role of periods of multiple disturbance events. J Trop Ecol 20:131–143
Newbery DM, Chuyong GB, Zimmerman L (2006a) Mast fruiting of large ectomycorrhizal African rain forest trees: importance of dry season intensity, and the resource-limitation hypothesis. New Phytol 170:561–579
Newbery DM, Chuyong GB, Zimmerman L, Praz C (2006b) Seedling survival and growth of three ectomycorrhizal caesalpiniaceous tree species in a Central African rain forest. J Trop Ecol 22:499–511
Newbery DM, Schwan S, Chuyong GB, van der Burgt XM (2009) Buttress form of the central African rain forest tree Microberlinia bisulcata, and its possible role in nutrient acquisition. Trees-Struct Funct 23:219–234
Newbery DM, Praz CJ, van der Burgt XM, Norghauer JM, Chuyong GB (2010) Recruitment dynamics of the grove-dominant tree Microberlinia bisulcata in African rain forest: extending the light response versus adult longevity trade-off concept. Plant Ecol 206:151–172
Newbery DM, van der Burgt XM, Worbes M, Chuyong GB (2013) Transient dominance in a central African rain forest. Ecol Monogr 83:339–382
Norghauer JM, Newbery DM (2010) Recruitment limitation after mast-seeding in two African rain forest trees. Ecology 91:2303–2312
Norghauer JM, Newbery DM (2011) Seed fate and seedling dynamics after masting in two African rain forest trees. Ecol Monogr 81:443–468
Norghauer JM, Newbery DM (2013) Herbivores equalize the seedling height growth of three dominant tree species in an African tropical rain forest. For Ecol Manag 310:555–566
Norghauer JM, Newbery DM (2015) Tree size and fecundity influence ballistic seed dispersal of two dominant mast-fruiting species in a tropical rain forest. For Ecol Manag 338:100–113
Norghauer JM, Newbery DM, Tedersoo L, Chuyong GB (2010) Do fungal pathogens drive density-dependent mortality in established seedlings of two dominant African rain forest trees? J Trop Ecol 26:293–301
Norghauer JM, Glauser G, Newbery DM (2014) Seedling resistance, tolerance and escape from herbivores: insights from co-dominant canopy tree species in a resource-poor African rain forest. Funct Ecol 28:1426–1439
Onguene NA, Kuyper TW (2002) Importance of the ectomycorrhizal network for seedling survival and ectomycorrhiza formation in rain forests of south Cameroon. Mycorrhiza 12:13–17
Paine CET, Harms KE, Schnitzer SA, Carson WP (2008) Weak competition among tropical tree seedlings: implications for species coexistence. Biotropica 40:432–440
Pinero D, Martinez-Ramos M, Sarukhan J (1984) A population-model of Astrocaryum mexicanum and a sensitivity analysis of its finite rate of increase. J Ecol 72:977–991
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113
Seidler TG, Plotkin JB (2006) Seed dispersal and spatial pattern in tropical trees. PLoS Biol 4:2132–2137
Selosse MA, Richard F, He X, Simard SW (2006) Mycorrhizal networks: des liaisons dangereuses? Trends Ecol Evol 21:621–628
Sibly RM, Hone J (2002) Population growth rate and its determinants: an overview. Philos Trans R Soc B 357:1153–1170
Silvertown JW (1980) The evolutionary ecology of mast seeding in trees. Biol J Linnean Soc 14:235–250
Simard SW, Durall DM (2004) Mycorrhizal networks: a review of their extent, function, and importance. Can J Bot 82:1140–1165
Svenning JC, Fabbro T, Wright SJ (2008) Seedling interactions in a tropical forest in Panama. Oecologia 155:143–150
Teste FP, Simard SW, Durall DM, Guy RD, Jones MD, Schoonmaker AL (2009) Access to mycorrhizal networks and roots of trees: importance for seedling survival and resource transfer. Ecology 90:2808–2822
Turner IM (2001) The ecology of trees in the tropical rain forest. Cambridge University Press, Cambridge
Vandermeer JH, Goldberg DE (2003) Population ecology: first principles. Princeton University Press, Princeton
Watkinson AR (1986) Plant population dynamics. In: Crawley M (ed) Plant ecology. Blackwell Scientific Publications, Oxford, pp 137–184
Weiner J (1988) Variation in the performance of individuals in plant populations. In: Davy AJ, Hutchings MJ, Watkinson AR (eds) Plant population ecology. Blackwell Scientific Publications, Oxford, pp 59–81
Wills C, Condit R (1999) Similar non-random processes maintain diversity in two tropical rainforests. Proc R Soc B 266:1445–1452
Wills C, Harms KE, Condit R, King D, Thompson J, He FL, Muller-Landau HC, Ashton P, Losos E, Comita L, Hubbell S, LaFrankie J, Bunyavejchewin S, Dattaraja HS, Davies S, Esufali S, Foster R, Gunatilleke N, Gunatilleke S, Hall P, Itoh A, John R, Kiratiprayoon S, de Lao SL, Massa M, Nath C, Noor MNS, Kassim AR, Sukumar R, Suresh HS, Sun IF, Tan S, Yamakura T, Zimmerman J (2006) Nonrandom processes maintain diversity in tropical rainforests. Science 311:527–531
Wright SJ (2002) Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130:1–14
Zhu K, Woodall CW, Monteiro JVD, Clark JS (2015) Prevalence and strength of density-dependent tree recruitment. Ecology 96:2319–2327
Zimmerman JK, Thompson J, Brokaw N (2008) Large tropical forest dynamics plots: testing explanations for the maintenance of diversity. In: Carson WP, Schnitzer SA (eds) Tropical forest community ecology. Blackwell Publishing, Oxford, pp 98–117
Zuidema PA, Brienen RJW, During HJ, Gueneralp B (2009) Do persistently fast-growing juveniles contribute disproportionately to population growth? A new analysis tool for matrix models and its application to rainforest trees. Am Nat 174:709–719
Acknowledgments
We thank S. Njibile and C. Okha for their outstanding assistance in the forest with data collection. We are grateful for support from previous Conservators of Korup National Park, A. Kembou and P. Ndongmo, and support from the Ministries of Forestry and Wildlife (MINFOF) and Scientific Research and Innovation (MINRESI) in Cameroon for research permission. G.B. Chuyong and R. Kometa of the University of Buea provided invaluable logistic support. The field research reported here complied with the current laws of country (Cameroon) in which it was carried out.
Author contribution statement
JMN and DMN conceived and designed the study. JMN conducted the field work to collect the data. JMN carried out the statistical analyses. JMN and DMN interpreted the analyses and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Katherine L. Gross.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Norghauer, J.M., Newbery, D.M. Density-dependent dynamics of a dominant rain forest tree change with juvenile stage and time of masting. Oecologia 181, 207–223 (2016). https://doi.org/10.1007/s00442-015-3534-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3534-9