Abstract
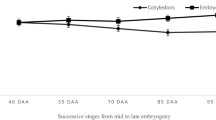
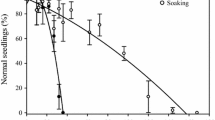
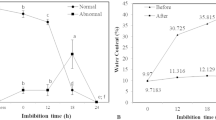
The acquisition of desiccation tolerance (DT) in developing beech (Fagus sylvatica L.) seeds and the role of a dehydrin protein in this process were investigated. DT was determined by measurement of electrolyte leakage and germination capacity after drying to 10–12% moisture content (MC). In addition to mass maturity, the presence of heat-stable proteins, dehydrin accumulation and the peak of ABA content were measured in relation to the acquisition of DT. Mass maturity was achieved at 16 weeks after flowering (WAF). The germination capacity increased from 8% at 12 WAF to 80–90% after 16 WAF. Cell membrane integrity, measured as a decrease in electrolyte leakage after desiccation, was acquired at 16 WAF. Additionally, the ratio of heat-stable to soluble proteins was the highest at 16 WAF. One dehydrin-like protein with a molecular mass 44 kDa, named DHN44, was detected in embryonic axes at 16 WAF and in cotyledons at 17 WAF, and its gradual accumulation was observed in mature seeds. With regard to the acquisition of DT, the strongest correlations were detected between electrolyte leakage, DHN44 accumulation, and the percentage of heat-stable proteins. These results suggest that developing beech seeds become tolerant to desiccation at 16 WAF. The effect of desiccation and ABA treatment on DHN44 synthesis was tested before (14 WAF) and after the DT acquisition (18 WAF). Depending on the maturation stage desiccation and ABA treatment can induce or enlarge DHN44 expression.







Similar content being viewed by others
References
Baker J, Steele C, Dure LIII (1988) Sequence and characterization of 6 Lea proteins and their genes from cotton. Plant Mol Biol 11:277–291. doi:10.1007/BF00027385
Black M, Corbineau F, Gee H, Côme D (1999) Water content, raffinose, and dehydrins in the induction of desiccation tolerance in immature wheat embryos. Plant Physiol 120:463–471. doi:10.1104/pp.120.2.463
Blackman SA, Obendorf RL, Leopold AC (1995) Desiccation tolerance in developing soybean seeds: the role of stress proteins. Physiol Plant 93(4):630–638. doi:10.1111/j.1399-3054.1995.tb05110.x
Blackman SA, Wettlaufer SH, Obendorf RL, Leopold AC (1991) Maturation proteins associated with desiccation tolerance in soybean. Plant Physiol 96(3):868–874
Bonner FT (1990) Storage of seeds: potential and limitations for germplasm conservation. For Ecol Manage 35:35–43
Bostock RM, Quatrano RS (1992) Regulation of Em gene expression in rice. Plant Physiol 98:1356–1363
Boudet J, Buitink J, Hoekstra FA, Rogniaux H, Larré C, Satour P et al (2006) Comparative analysis of the heat-stable proteome of radicles of Medicago truncatula seeds during germination identifies late embryogenesis abundant proteins associated with desiccation tolerance. Plant Physiol 140(4):1418–1436. doi:10.1104/pp.105.074039
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities utilizing the principle of protein dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Buitink J, Vu BL, Satour P, Leprince O (2003) The re-establishment of desiccation tolerance in germinated radicles of Medicago truncatula Gaernt. seeds. Seed Sci Res 13:273–286. doi:10.1079/SSR2003145
Close TJ (1996) Dehydrins: emergence of a biochemical role of a family of plant dehydration proteins. Physiol Plant 97:795–803. doi:10.1111/j.1399-3054.1996.tb00546.x
Close TJ (1997) Dehydrins: a commonalty in the response of plants to dehydration and low temperature. Physiol Plant 100(2):291–296. doi:10.1111/j.1399-3054.1997.tb04785.x
Close TJ, Fenton RD, Moonan F (1993) A view of plant dehydrins using antibodies specific to the carboxy terminal peptide. Plant Mol Biol 23(2):279–286. doi:10.1007/BF00029004
Daws MI, Lydall E, Chmielarz P, Leprince O, Mathews S, Thanos CA et al (2004) Developmental heat sum influences recalcitrant seed traits in Aesculus hippocastanum across Europe. New Phytol 162:157–166. doi:10.1111/j.1469-8137.2004.01012.x
Dure LIII (1993) A repeating 11-mer amino acid motif and plant desiccation. Plant J 3(3):363–369. doi:10.1046/j.1365-313X.1993.t01-18-00999.x
Ellis RH, Hong TD, Roberts EH (1990) An intermediate category of seed storage behaviour? J Exp Bot 41:1167–1174. doi:10.1093/jxb/41.9.1167
Ellis RH, Hong TD, Roberts EH (1991) An intermediate category of seed storage behaviour? II: effects of provenance, immaturity, and imbibition on desiccation-tolerance in coffee. J Exp Bot 42:653–657. doi:10.1093/jxb/42.5.653
Ellis RH, Pieta Filho C (1992) Seed development and cereal seed longevity. Seed Sci Res 2:9–15
Fu JR, Yang XQ, Jiang XC, He JX, Song SQ (1997) Heat stable proteins and desiccation tolerance in recalcitrant and orthodox seeds. In: Ellis RH, Black M, Murdoch AJ, Hong TD (eds) Basic and applied aspects of seeds biology, Kluwer, Dordrecht, pp 705–713
Goldberg RB, de Paiva G, Yadegari R (1994) Plant embryogenesis: zygote to seed. Science 266(5185):605–614. doi:10.1126/science.266.5185.605
Gosling PG (1991) Beechnuts storage: a review and practical interpretation of the scientific literature. Forestry 64:51–59. doi:10.1093/forestry/64.1.51
Goyal K, Walton LJ, Tunnacliffe A (2005) LEA proteins prevent protein aggregation due to water stress. Biochem J 388:151–157. doi:10.1042/BJ20041931
Han B, Berjak P, Pammenter N, Farrant J, Kermode AR (1997) The recalcitrant plant species, Castanospermum australe and Trichilia dregeana, differ in their ability to produce dehydrin-related polypeptides during seed maturation and in response to ABA or water-deficit-related stresses. J Exp Bot 48(9):1717–1726. doi:10.1093/jxb/48.9.1717
Hinniger C, Caillet V, Michoux F, Ben Amor M, Tanksley S, Lin C et al (2006) Isolation and characterization of cDNA encoding three dehydrins expressed during Coffea canephora (Robusta) grain development. Ann Bot (Lond) 97(5):755–765. doi:10.1093/aob/mcl032
Hoekstra FA, Golovina EA, Buitink J (2001) Mechanisms of plant desiccation tolerance. Trends Plant Sci 6(9):431–438. doi:10.1016/S1360-1385(01)02052-0
Ismail AM, Hall AE, Close TJ (1999) Purification and partial characterization of a dehydrin involved in chilling tolerance during seedling emergence of cowpea. Plant Physiol 120:237–244. doi:10.1104/pp.120.1.237
ISTA (1999) International rules for seed testing: rules1999. Seed Sci Technol 27(Suppl):1–133
Jiménez JA, Rodríguez D, Lorenzo O, Nicolás G, Nicolás C (2006) Characterization of a protein kinase (FsPK4) with an acidic domain, regulated by abscisic acid and specifically located in Fagus sylvatica L. seeds. J Plant Physiol 163:761–769. doi:10.1016/j.jplph.2005.07.010
Jiménez JA, Alonso-Ramírez A, Nicolás C (2008) Two cDNA clones (FsDhn1 and FsClo1) up regulated by ABA are involved in drought responses in Fagus sylvatica L. seeds. J Plant Physiol (Epub ahead of print)
Kalemba EM, Pukacka S (2008) Changes in late embryogenesis abundant proteins and a small heat shock protein during storage of beech (Fagus sylvatica L.) seeds. Environ Exp Bot 63:274–280. doi:10.1016/j.envexpbot.2007.12.011
Kermode AR, Bewley JD (1985) The role of maturation drying in the transition from seed development to germination. J Exp Bot 36(12):1916–1927. doi:10.1093/jxb/36.12.1916
Kermode AR, Bewley JD, Dasgupta J, Misra S (1986) The transition from seed development to germination: a key role for desiccation? HortScience 21(5):1113–1118
Kermode AR (1997) Approaches to elucidate the basis of desiccation-tolerance in seeds. Seed Sci Res 7:75–95
Kermode AR (2005) Role of abscisic acid in seed dormancy. J Plant Growth Regul 24:319–344. doi:10.1007/s00344-005-0110-2
Koornneef M, Hanhart CJ, Hilhorst HWM, Karssen CM (1989) In vivo inhibition of seed development and reserve protein accumulation in recombinants of abscisic acid biosynthesis and responsiveness mutants in Arabidopsis thaliana. Plant Physiol 90:463–469
Koster KL, Leopold AC (1988) Sugars and desiccation tolerance in seeds. Plant Physiol 88(3):829–832
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi:10.1038/227680a0
Lane BG (1991) Cellular desiccation and hydration: developmentally regulated proteins, and the maturation and germination of seed embryos. FASEB J 5:2893–2901
Le Page-Degivry MT, Garello G, Barthe P (1997) Changes in abscisic acid biosynthesis and catabolism during dormancy breaking in Fagus sylvatica embryo. J Plant Growth Regul 16:57–61. doi:10.1007/PL00006978
León-Lobos P, Ellis RH (2002) Seed storage behaviour of Fagus sylvatica and Fagus crenata. Seed Sci Res 12:31–37. doi:10.1079/SSR200195
Liang Y, Sun WQ (2000) Desiccation tolerance of recalcitrant Theobroma cacao embryonic axes: the optimal drying rate and its physiological basis. J Exp Bot 51(352):1911–1919. doi:10.1093/jexbot/51.352.1911
Marian CO, Krebs SL, Arora R (2003) Dehydrin variability among rhododendron species: a 25-kDa dehydrin is conserved and associated with cold acclimation across diverse species. New Phytol 161:773–780. doi:10.1111/j.1469-8137.2003.01001.x
Mundy J, Chuan H (1988) Abscisic acid and water stress induce the expression of a novel gene. EMBO J 7:2279–2286
Nicolás C, Rodríguez D, Poulsen F, Eriksen EN, Nicolás G (1997) The expression of an abscisic acid-responsive glycine-rich protein coincides with the level of seed dormancy in Fagus sylvatica. Plant Cell Physiol 38:1303–1310
Panza V, Distéfano AJ, Carjuzaa P, Láinez V, Del Vas M, Maldonado S (2007) Detection of dehydrin-like proteins in embryos and endosperm of mature Euterpe edulis seeds. Protoplasma 231:1–5. doi:10.1007/s00709-007-0248-9
Pawlowski TA (2007) Proteomics of European beech (Fagus sylvatica L.) seed dormancy breaking: influence of abscisic and gibberellic acids. Proteomics 7:2246–2257. doi:10.1002/pmic.200600912
Poulsen KM (1993) Predicting the storage life of beechnuts. Seed Sci Technol 21:327–337
Poulsen KM, Kundsen H (1999) Viability constants based on eight years storage of beech nuts (Fagus sylvatica L.). Seed Sci Technol 27:1037–1039
Pukacka S, Hoffmann SK, Goslar J, Pukacki PM, Wójkiewicz E (2003) Water and lipid relations in beech (Fagus sylvatica L.) seeds and its effect on storage behaviour. Biochim Biophys Acta 1621(1):48–56
Pukacka S, Wójkiewicz E (2003) The effect of temperature of drying on viability and some factors affecting storability of Fagus sylvatica seeds. Acta Physiol Plant 25:163–169. doi:10.1007/s11738-003-0049-5
Pukacka S, Ratajczak E (2007a) Ascorbate and glutathione metabolism during development and desiccation of orthodox and recalcitrant seeds of the genus Acer. Funct Plant Biol 34:601–613. doi:10.1071/FP07013
Pukacka S, Ratajczak E (2007b) Age-related biochemical changes during storage of beech (Fagus sylvatica L.) seeds. Seed Sci Res 17:45–53. doi:10.1017/S0960258507629432
Reyes D, Rodríguez D, González-García MP, Lorenzo O, Nicolás G, García-Martínez JL et al (2006a) Overexpression of a protein phosphatase 2C from beech seeds in Arabidopsis shows phenotypes related to abscisic acid responses and gibberellin biosynthesis. Plant Physiol 141:1414–1421. doi:10.1104/pp.106.084681
Reyes D, Rodríguez D, Lorenzo O, Nicolás G, Cañas R, Cantón FR et al (2006b) Immunolocalization of FsPK1 correlates this abscisic acid-induced protein kinase with germination arrest in Fagus sylvatica L. seeds. J Exp Bot 57:923–929. doi:10.1093/jxb/erj077
Roberts EH (1973) Predicting the storage life of seeds. Seed Sci Technol 1:499–514
Rorat T (2006) Plant dehydrins–tissue location, structure and function. Cell Mol Biol Lett 11:536–556. doi:10.2478/s11658-006-0044-0
Sanhewe AJ, Ellis RH (1996) Seed development and maturation in Phaseolus vulgaris II: post-harvest longevity in air-dry storage. J Exp Bot 47(7):959–965. doi:10.1093/jxb/47.7.959
Shen Q, Zhang P, Ho THD (1996) Modular nature of abscisic acid (ABA) response complexes: composite promoter units that are necessary and sufficient for aba lnduction of gene expression in barley. Plant Cell 8:1107–1119
Sun WQ, Leopold AC (1993) Acquisition of desiccation tolerance in soybeans. Physiol Plant 87(3):403–409. doi:10.1111/j.1399-3054.1993.tb01748.x
Suszka B, Muller C, Bonnet-Masimbert M (1994) Seeds of forest trees: from collection to seedlings. INRA, Paris
Walker-Simmons MK, Abrams SR (1991) Use of ABA immunoassays. In: Davies WJ, Jones HG (eds) Abscisic acid: physiology and biochemistry: environmental plant biology series. Bios-Scientific Publishers, Oxford, pp 53–79
Williamson JD, Quatrano RS (1988) ABA-regulation of two classes of embryospecific sequences in mature wheat embryos. Plant Physiol 86:208–215
Wise MJ (2003) LEAping to conclusions: a computational reanalysis of late embryogenesis abundant proteins and their possible roles. BMC Bioinformatics 4:52. doi:10.1186/1471-2105-4-52
Xu D, Duan X, Wang B, Hong B, Ho THD, Wu R (1996) Expression of a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol 110:249–257
Acknowledgments
The antiserum raised against the K segment was kindly supplied by T. J. Close, Department of Botany and Plant Sciences, University of California, Riverside, CA, USA. This research was supported by The Ministry of Science and Information (Poland)—grant No. 2PO6L02927.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Major.
Rights and permissions
About this article
Cite this article
Kalemba, E.M., Janowiak, F. & Pukacka, S. Desiccation tolerance acquisition in developing beech (Fagus sylvatica L.) seeds: the contribution of dehydrin-like protein. Trees 23, 305–315 (2009). https://doi.org/10.1007/s00468-008-0278-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-008-0278-8