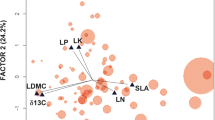
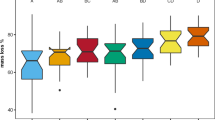
Abstract
Key message
There is a positive correlation between leaf and root decomposition across species, both in a warm-temperate forest in Japan, as well as globally.
Abstract
Evaluating the effects of plant species traits on litter decomposition would increase our understanding of plant–soil feedbacks in forest ecosystems. Currently, an assessment of a possible coordination between leaf and root decomposition across different species is required. However, previous studies have generated conflicting results. We hypothesized that such inconsistencies may be attributed to differences in local climatic effects on the decomposition process. We focused on the linkages between leaf and fine-root decomposition of woody species in a warm-temperate forest, which have not been addressed in previous studies. We found a significant positive correlation between leaf and root decomposition, and this linkage may be attributed to a wider range of decomposition rates across the species in our study forest. Additionally, we combined our data with those of previous studies of woody species to infer a global linkage in the decomposition process between leaves and roots. We found a positive correlation in decomposition rates between leaves and roots at the global scale, as well as a relatively strong correlation in warmer regions. These results support the importance of litter quality on biogeochemical processes and suggest that synergetic interactions between climate and plant communities could be amplified in a warmer future.



Similar content being viewed by others
References
Adams MB, Campbell RG, Allen HL, Davey CB (1987) Root and foliar nutrient concentrations in loblolly pine: effects of season, site, and fertilization. For Sci 33:984–996
Aerts R (1990) Nutrient use efficiency in evergreen and deciduous species from heathlands. Oecologia 84:391–397
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449. doi:10.2307/3546886
Aulen M, Shipley B, Bradley R (2012) Prediction of in situ root decomposition rates in an interspecific context from chemical and morphological traits. Ann Bot 109:287–297. doi:10.1093/aob/mcr259
Bardgett RD, Wardle DA (2010) Aboveground–belowground linkages: biotic interactions, ecosystem processes, and global change. Oxford University Press, New York
Berg B, McClaugherty CA (2008) Plant litter, decomposition, humus formation, carbon sequestration, second edn. Springer-Verlag, Berlin
Berg B et al (1993) Litter mass loss rates in pine forests of Europe and Eastern United States: some relationships with climate and litter quality. Biogeochemistry 20:127–159
Birouste M, Kazakou E, Blanchard A, Roumet C (2012) Plant traits and decomposition: are the relationships for roots comparable to those for leaves? Ann Bot 109:463–472. doi:10.1093/aob/mcr297
Chapin FS III, Matson PA, Vitousek PM (2011) Principles of terrestrial ecosystem ecology. Springer, New York
Cornelissen JHC (1996) An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. J Ecol 84:573–582. doi:10.2307/2261479
Cornelissen JHC, Thompson K (1997) Functional leaf attributes predict litter decomposition rate in herbaceous plants. New Phytol 135:109–114
Cornwell WK et al (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071. doi:10.1111/j.1461-0248.2008.01219.x
Couteaux M-M, Bottner P, Berg B (1995) Litter decomposition, climate and litter quality. Trends Ecol Evol 10:63–66
Craine JM (2009) Resource strategies of wild plants. Princeton University Press, Princeton
Craine JM, Lee WG, Bond WJ, Williamas RJ, Johnson LC (2005) Environmental constraints on a global relationship among leaf and root traits of grasses. Ecology 86:12–19
Esau K (1964) Plant anatomy, 2nd edn. Wiley, New York
Fan P, Guo D (2010) Slow decomposition of lower order roots: a key mechanism of root carbon and nutrient retention in the soil. Oecologia 163:509–515. doi:10.1007/s00442-009-1541-4
Fortunel C et al (2009) Leaf traits capture the effects of land use changes and climate on litter decomposability of grasslands across Europe. Ecology 90:598–611
Freschet GT, Cornelissen JH, van Logtestijn RS, Aerts R (2010a) Substantial nutrient resorption from leaves, stems and roots in a subarctic flora: what is the link with other resource economics traits? New Phytol 186:879–889. doi:10.1111/j.1469-8137.2010.03228.x
Freschet GT, Cornelissen JHC, van Logtestijn RSP, Aerts R (2010b) Evidence of the ‘plant economics spectrum’ in a subarctic flora. J Ecol 98:362–373. doi:10.1111/j.1365-2745.2009.01615.x
Freschet GT, Aerts R, Cornelissen JHC (2012) A plant economics spectrum of litter decomposability. Funct Ecol 26:56–65. doi:10.1111/j.1365-2435.2011.01913.x
Freschet GT et al (2013) Linking litter decomposition of above- and below-ground organs to plant–soil feedbacks worldwide. J Ecol 101:943–952. doi:10.1111/1365-2745.12092
Fujii S, Takeda H (2010) Dominant effects of litter substrate quality on the difference between leaf and root decomposition process above- and belowground. Soil Biol Biochem 42:2224–2230. doi:10.1016/j.soilbio.2010.08.022
Fujimaki R (2005) Mechanism and function of fine root production in forest ecosystems. Doctoral thesis, Kyoto University (Doctoral Thesis)
Goebel M et al (2011) Decomposition of the finest root branching orders: linking belowground dynamics to fine-root function and structure. Ecol Monogr 81:89–102. doi:10.1890/09-2390.1
Graaff M-Ad, Six J, Jastrow JD, Schadt CW, Wullschleger SD (2013) Variation in root architecture among switchgrass cultivars impacts root decomposition rates. Soil Biol Biochem 58:198–206. doi:10.1016/j.soilbio.2012.11.015
Hishi T (2007) Heterogeneity of individual roots within the fine root architecture: causal links between physiological and ecosystem functions. Journal of Forest Research 12:126–133. doi:10.1007/s10310-006-0260-5
Hobbie SE (1992) Effects of plant species on nutrient cycling. Trends Ecol Evol 7:336–339. doi:10.1016/0169-5347(92)90126-V
Hobbie SE, Oleksyn J, Eissenstat DM, Reich PB (2010) Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia 162:505–513. doi:10.1007/s00442-009-1479-6
Holdaway RJ, Richardson SJ, Dickie IA, Peltzer DA, Coomes DA (2011) Species- and community-level patterns in fine root traits along a 120,000-year soil chronosequence in temperate rain forest. J Ecol 99:954–963. doi:10.1111/j.1365-2745.2011.01821.x
IUSS Working Group WRB (2006) World reference base for soil resources 2006. FAO, Rome
Kerkhoff AJ, Fagan WF, Elser JJ, Enquist BJ (2006) Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am Nat 168:E103–E122
Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology 77:1716–1727
Lavelle P, Blanchart E, Martin A, Martin S, Spain A (1993) A hierarchical model for decomposition in terrestrial ecosystems: application to Soils of the Humid Tropics. Biotropica 25:130. doi:10.2307/2389178
Morishita K, Ando M (2002) Change in cover types of urban forests damaged by pine wilt disease in the northern part of Kyoto City. Forest Research (in Japanese) 74:35–45
Norby RJ, Ledford J, Reilly CD, Miller NE, O’Neill EG (2004) Fine-root production dominates response of a deciduous forest to atmospheric CO2 enrichment. Proc Natl Acad Sci USA 101:9689–9693. doi:10.1073/pnas.0403491101
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Park BB, Yanai RD (2009) Nutrient concentrations in roots, leaves and wood of seedling and mature sugar maple and American beech at two contrasting sites. For Ecol Manage 258:1153–1160. doi:10.1016/j.foreco.2009.06.003
Perez-Harguindeguy N, Dıaz S, Cornelissen JHC, Vendramini F, Cabido M, Castellanos A (2000) Chemistry and toughness predict leaf litter decomposition rates over a wide spectrum of functional types and taxa in central Argentina. Plant Soil 218:21–30
Pregitzer KS, DeForest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL (2002) Fine root architecture of nine North American trees. Ecol Monogr 72:293–309. doi:10.2307/3100029
Rasse DP, Rumpel C, Dignac M-F (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269:341–356. doi:10.1007/s11104-004-0907-y
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419. doi:10.1007/s004420100740
Smith MD, Knapp AK, Collins SL (2009) A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology 90:3279–3289
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. Blackwell Scientific Publications, Oxford, London
Takeda H, Ishida Y, Tsutsumi T (1987) Decomposition of leaf litter in relation to litter quality and site conditions. Memoirs of the College of Agriculture, Kyoto Univ 130:17–38
Tjoelker MG, Craine JM, Wedin D, Reich PB, Tilman D (2005) Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytol 167:493–508. doi:10.1111/j.1469-8137.2005.01428.x
Tsutsumi T (1973) Nutrient Production in terrestrial plant community, vol 1b. Lectures of Ecology, Kyoritsu Shuppan, Tokyo (in Japanese)
Wang H, Liu S, Mo J (2010) Correlation between leaf litter and fine root decomposition among subtropical tree species. Plant Soil 335:289–298. doi:10.1007/s11104-010-0415-1
Withington JM, Reich PB, Oleksyn J, Eissenstat DM (2006) Comparisons of structure and life span in roots and leaves among temperate trees. Ecol Monogr 76:381–397
Zhang D, Hui D, Luo Y, Zhou G (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 1:85–93. doi:10.1093/jpe/rtn002
Author contribution statement
SF and NM designed and conducted the experiment. SF and ASM analyzed the data. SF and ASM wrote the manuscript with critical inputs from NM and HT.
Acknowledgments
We thank Dr. Noriyuki Osada, Ms. Ayumi Kawamura Ms. Shoko Oguchi, and the staff of the Kamigamo Experimental Station, Field Science Education and Research Center of Kyoto University for their support of this study. This study was supported by the Fujiwara Natural History Foundation and by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Young Scientists (No. 25850115 to S.F.). We thank Dr. Ulrich Lüttge, guest Editor and anonymous reviewers for their helpful comments.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Koike and K. Noguchi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fujii, S., Makita, N., Mori, A.S. et al. A stronger coordination of litter decomposability between leaves and fine roots for woody species in a warmer region. Trees 30, 395–404 (2016). https://doi.org/10.1007/s00468-015-1221-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-015-1221-4