Abstract
Purpose
To assess changes in neutropenia-related hospitalization, myelosuppressive chemotherapy, and primary prophylactic colony-stimulating factor (PP-CSF) use in elderly cancer patients receiving myelosuppressive chemotherapy.
Methods
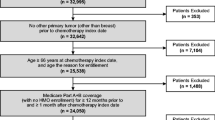
We identified annual cohorts of patients aged ≥ 66 years with breast cancer, lung cancer, or non-Hodgkin lymphoma (NHL) initiating myelosuppressive chemotherapy during 1995–2015 using Medicare 5% (1994–2008) and 20% (2007–2015) data. We described myelosuppressive chemotherapy changes by febrile neutropenia (FN) risk category (high, intermediate, unclassified), PP-CSF use, and, in the first cycle of myelosuppressive chemotherapy, neutropenia-related hospitalization (ICD-9-CM: 288.0X, first 5 positions). We evaluated hospitalization trends using a logistic regression model with spline curve of calendar year adjusting for baseline characteristics.
Results
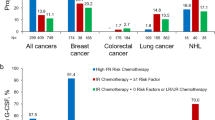
Annual cohorts included 1451–2114 eligible patients for 1995–2007 and 5272–7603 for 2008–2015. Myelosuppressive chemotherapy use with high/intermediate FN risk increased from 31% in 1995 to 56% in 1999, stabilized through 2008 (range 56–61%), then decreased to 52% in 2015. PP-CSF use increased from 5.5% in 1995 to 52.7% in 2015, mainly due to pegfilgrastim introduction in 2002. Crude neutropenia-related hospitalization incidence decreased from 5.2% in 1995 to 2.7% in 2015; adjusted incidence decreased, on average, by 4.7% yearly before 2010 (p < 0.0001) and was flat from 2010 onward (p = 0.53).
Conclusions
Among elderly patients with breast cancer, lung cancer, or NHL receiving myelosuppressive chemotherapy, PP-CSF use increased substantially after 2002. Neutropenia-related hospitalization incidence in the first cycle decreased yearly before 2010 and was flat afterward. Further studies are needed to understand overall decreasing neutropenia-related hospitalization trends and effects of changes in myelosuppressive chemotherapy and FN management.





Similar content being viewed by others
References
Smith RE (2006) Trends in recommendations for myelosuppressive chemotherapy for the treatment of solid tumors. J Natl Compr Cancer Netw 4:649–658
Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS (2012) Decline in the use of anthracyclines for breast cancer. J Clin Oncol 30:2232–2239
Marquart J, Chen EY, Prasad V (2018) Estimation of the percentage of us patients with cancer who benefit from genome-driven oncology. JAMA Oncol 4:1093–1098
Crawford J, Dale DC, Kuderer NM, Culakova E, Poniewierski MS, Wolff D, Lyman GH (2008) Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Cancer Netw 6:109–118
Lyman GH, Kuderer NM (2003) Epidemiology of febrile neutropenia. Support Cancer Ther 1:23–35
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258–2266
Lyman GH, Michels SL, Reynolds MW, Barron R, Tomic KS, Yu J (2010) Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer 116:5555–5563
Schilling MB, Parks C, Deeter RG (2011) Costs and outcomes associated with hospitalized cancer patients with neutropenic complications: a retrospective study. Exp Ther Med 2:859–866
Caggiano V, Weiss RV, Rickert TS, Linde-Zwirble WT (2005) Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer 103:1916–1924
Tai E, Guy GP Jr, Dunbar A, Richardson LC (2017) Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract 13:e552–e561
Li S, Liu J, Bowers C, Garawin T, Kim C, Bensink ME, Chandler DB (2019) Febrile neutropenia-related care and associated costs in elderly patients with breast cancer, lung cancer, or non-Hodgkin lymphoma. Support Care Cancer. https://doi.org/10.1007/s001090000086
Lyman GH, Dale DC, Crawford J (2003) Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol 21:4524–4531
Lyman GH, Dale DC, Friedberg J, Crawford J, Fisher RI (2004) Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: a nationwide study. J Clin Oncol 22:4302–4311
Lyman GH (2009) Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Cancer Netw 7:99–108
Shayne M, Culakova E, Poniewierski MS, Wolff D, Dale DC, Crawford J, Lyman GH (2007) Dose intensity and hematologic toxicity in older cancer patients receiving systemic chemotherapy. Cancer 110:1611–1620
Shayne M, Culakova E, Wolff D, Poniewierski MS, Dale DC, Crawford J, Lyman GH (2009) Dose intensity and hematologic toxicity in older breast cancer patients receiving systemic chemotherapy. Cancer 115:5319–5328
Mhaskar R, Clark OA, Lyman G, Engel Ayer Botrel T, Morganti Paladini L, Djulbegovic B (2014) Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst Rev CD003039
Kuderer NM, Dale DC, Crawford J, Lyman GH (2007) Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 25:3158–3167
Vogel CL, Wojtukiewicz MZ, Carroll RR, Tjulandin SA, Barajas-Figueroa LJ, Wiens BL, Neumann TA, Schwartzberg LS (2005) First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol 23:1178–1184
Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, Kris M, Grous J, Picozzi V, Rausch G et al (1991) Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 325:164–170
NCCN® Clinical Practice Guidelines in Oncology. Hematopoietic growth factors. V2.2019. National Comprehensive Cancer Network website. Available at: https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf. Accessed 30 May 2019
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, America IDSo (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52:427–431
Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, Goldberg JM, Khatcheressian JL, Leighl NB, Perkins CL, Somlo G, Wade JL, Wozniak AJ, Armitage JO, American Society of Clinical O (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33:3199–3212
Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C, European Organisation for R, Treatment of C (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32
Ramsey SD, McCune JS, Blough DK, McDermott CL, Clarke L, Malin JL, Sullivan SD (2010) Colony-stimulating factor prescribing patterns in patients receiving chemotherapy for cancer. Am J Manag Care 16:678–686
Sosa R, Li S, Molony JT, Liu J, Stryker S, Collins AJ (2017) Use of prophylactic growth factors and antimicrobials in elderly patients with cancer: a review of the Medicare database. Support Care Cancer 25:3123–3132
Elting LS, Xu Y, Chavez-MacGregor M, Giordano SH (2016) Granulocyte growth factor use in elderly patients with non-Hodgkin’s lymphoma in the United States: adherence to guidelines and comparative effectiveness. Support Care Cancer 24:2695–2706
Goyal RK, Tzivelekis S, Rothman KJ, Candrilli SD, Kaye JA (2018) Time trends in utilization of G-CSF prophylaxis and risk of febrile neutropenia in a Medicare population receiving adjuvant chemotherapy for early-stage breast cancer. Support Care Cancer 26:539–548
Mues KE, Liede A, Liu J, Wetmore JB, Zaha R, Bradbury BD, Collins AJ, Gilbertson DT (2017) Use of the Medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US. Clin Epidemiol 9:267–277
Weycker D, Li X, Tzivelekis S, Atwood M, Garcia J, Li Y, Reiner M, Lyman GH (2017) Burden of chemotherapy-induced febrile neutropenia hospitalizations in us clinical practice, by use and patterns of prophylaxis with colony-stimulating factor. Support Care Cancer 25:439–447
NCCN® Clinical Practice Guidelines in Oncology. Myeloid growth factors. National Comprehensive Cancer Network website. Available at: www.nccn.org. Accessed 30 May 2019
Crawford J, Becker PS, Armitage JO, Blayney DW, Chavez J, Curtin P, Dinner S, Fynan T, Gojo I, Griffiths EA, Hough S, Kloth DD, Kuter DJ, Lyman GH, Mably M, Mukherjee S, Patel S, Perez LE, Poust A, Rampal R, Roy V, Rugo HS, Saad AA, Schwartzberg LS, Shayani S, Talbott M, Vadhan-Raj S, Vasu S, Wadleigh M, Westervelt P, Burns JL, Pluchino L (2017) Myeloid growth factors, Version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 15:1520–1541
Weycker D, Sofrygin O, Seefeld K, Deeter RG, Legg J, Edelsberg J (2013) Technical evaluation of methods for identifying chemotherapy-induced febrile neutropenia in healthcare claims databases. BMC Health Serv Res 13:60
Li Y, Klippel Z, Shih X, Reiner M, Wang H, Page JH (2016) Relationship between severity and duration of chemotherapy-induced neutropenia and risk of infection among patients with nonmyeloid malignancies. Support Care Cancer 24:4377–4383
Lyman GH, Lyman CH, Agboola O (2005) Risk models for predicting chemotherapy-induced neutropenia. Oncologist 10:427–437
Du XL, Zhang Y, Hardy D (2016) Temporal and geographic variations in the receipt of colony-stimulating factors and erythropoiesis-stimulating agents in a large retrospective cohort of older women with breast cancer from 2000 to 2009. Am J Ther 23:e411–e421
Davies J, Patel M, Gridelli C, de Marinis F, Waterkamp D, McCusker ME (2017) Real-world treatment patterns for patients receiving second-line and third-line treatment for advanced non-small cell lung cancer: a systematic review of recently published studies. PLoS One 12:e0175679
Ho C, Ramsden K, Zhai Y, Murray N, Sun S, Melosky B, Laskin J (2014) Less toxic chemotherapy improves uptake of all lines of chemotherapy in advanced non-small-cell lung cancer: a 10-year retrospective population-based review. J Thorac Oncol 9:1180–1186
Kozma CM, Dickson M, Chia V, Legg J, Barron R (2012) Trends in neutropenia-related inpatient events. J Oncol Pract 8:149–155
Cuyún Carter G, Barrett AM, Kaye JA, Liepa AM, Winfree KB, John WJ (2014) A comprehensive review of nongenetic prognostic and predictive factors influencing the heterogeneity of outcomes in advanced non-small-cell lung cancer. Cancer Manag Res 6:437–449
Acknowledgments
Medical writing support was provided by Martha Mutomba (on behalf of Amgen Inc.). The authors thank Chronic Disease Research Group colleague Nan Booth for manuscript editing.
Funding
This study was financially supported by the Amgen Inc.
Author information
Authors and Affiliations
Contributions
Research idea and study design: SL, JL, PG, CK, MB, DC; data acquisition: SL, JL, HG; data analysis/interpretation: HG, JL, SL, PG, CK, MB, DC; statistical analysis: HG, JL; supervision or mentorship: SL, JL. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work.
Corresponding author
Ethics declarations
Conflict of interest
Shuling Li, Jiannong Liu, and Haifeng Guo are employees of Chronic Disease Research Group, Hennepin Healthcare Research Institute, which has received project funding from Amgen Inc. Prasad L. Gawade, Christopher Kim, Mark E. Bensink, and David Chandler are employees of and own stock in Amgen Inc.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Formal consent was not required as the article does not contain any studies involving human participants performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 404 kb)
Rights and permissions
About this article
Cite this article
Li, S., Liu, J., Guo, H. et al. Trends in the use of primary prophylactic colony-stimulating factors and neutropenia-related hospitalization in elderly cancer patients receiving myelosuppressive chemotherapy in the USA: 1995–2015. Support Care Cancer 28, 2637–2649 (2020). https://doi.org/10.1007/s00520-019-05080-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-05080-w