Abstract
Background
Obesity and weight gain have been associated with poor disease-specific and health-related outcomes in breast cancer survivors (BCS). But the benefits of weight loss in managing BCS have not been elucidated.
Objective
To evaluate the beneficial effects of weight loss programs in randomized controlled trials on BCS.
Methods
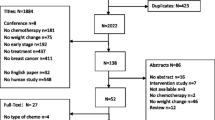
We searched English databases PubMed, the Cochrane Library, EMBASE, Scopus, Web of Science, CINAHL, and Chinese databases China National Knowledge Infrastructure (CNKI), Weipu Information Chinese Periodical Service Platform (VIP), China Biomedical Literature Service System (SinoMed), and Wanfang from the inception to January 2021 and collected randomized controlled trials (RCTs) of weight loss programs for BCS. Two reviewers independently screened the literature, extracted the data, and assessed the risk of bias in the included studies. The data synthesis was performed on RevMan (version 5.3), and the publication bias was calculated with STATA (version 15.1).
Results
Ten RCTs were included in the meta-analysis. The current study showed that diet and exercise interventions resulted in significant improvements in body weight (MD = − 4.43 kg, 95%CI: − 6.23 to − 2.63, P < 0.00001), waist circumference (MD = − 2.81 cm, 95%CI: − 4.37 to − 1.26, P = 0.004), hip circumference (MD = − 3.01 cm, 95%CI: − 4.24 to − 1.77, P < 0.0001), body mass index (MD = − 1.69 kg/m2, 95%CI: − 2.16 to − 1.21, P < 0.00001), systolic blood pressure (MD = − 12.12 mmHg, 95%CI: − 18.97 to − 5.27), p = 0.0005), C-reactive protein (MD = − 1.83 mg/L, 95% CI: − 2.74 to − 0.91, p < 0.0001), body fat (MD = − 1.19 kg, 95%CI: − 1.75 to − 0.63, P < 0.001), fat mass (MD = − 2.29 kg, 95%CI: − 3.12 to − 1.46, P < 0.0001), and lean body mass (MD = − 2.15 kg, 95%CI: − 3.66 to − 0.65, P = 0.005). Alternatively, compared with the effects of control interventions, weight loss programs did not affect fat-free mass, total cholesterol, low-density leptin cholesterol, glucose, insulin, and leptin (P > 0.05).
Conclusions
This review summarizes the benefits of weight loss programs for BCS. The results indicated that weight loss programs could significantly improve specific anthropometric outcomes but not affect biochemical indicators. Researchers should tailor weight loss interventions to the body fat status of BCS. Evidence to support the translation of effective weight loss intervention programs into wider-scale implementation is needed to be part of routine survivorship care.




Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- BCS:
-
Breast cancer survivors
- MD:
-
Mean difference
- SMD:
-
Standardized mean difference
- 95% CI:
-
95% Confidence interval
- RCTs:
-
Randomized controlled trials
- BMI:
-
Body mass index
- SBP:
-
Systolic blood pressure
- CRP:
-
C-reactive protein
- LDL-C:
-
Low-density leptin cholesterol
- TC:
-
Total cholesterol
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Makari-Judson G, Braun B, Jerry DJ et al (2014) Weight gain following breast cancer diagnosis: implication and proposed mechanisms. World J Clin Oncol 5(3):272–282. https://doi.org/10.5306/wjco.v5.i3.272
Sedjo RL, Byers T, Ganz PA et al (2014) Weight gain prior to entry into a weight-loss intervention study among overweight and obese breast cancer survivors. J Cancer Surviv 8(3):410–418. https://doi.org/10.1007/s11764-014-0351-9
Vagenas D, DiSipio T, Battistutta D, et al. Weight and weight change following breast cancer: evidence from a prospective, population-based, breast cancer cohort study. BMC Cancer. 2015;15:28. Published 2015 Jan 31. https://doi.org/10.1186/s12885-015-1026-2
Kim A, Scharf K, Senthil M et al (2014) The prevalence of overweight and obesity in a breast clinic population: consideration for weight loss as a therapeutic intervention. Surgery for Obesity and Related Diseases 10(2):348–353. https://doi.org/10.1016/j.soard.2013.07.019
Imayama I, Alfano CM, Neuhouser ML et al (2013) Weight, inflammation, cancer-related symptoms and health-related quality of life among breast cancer survivors. Breast Cancer Res Treat 140(1):159–176. https://doi.org/10.1007/s10549-013-2594-y
Demark-Wahnefried W, Campbell KL, Hayes SC (2012) Weight management and its role in breast cancer rehabilitation. Cancer 118:2277–2287. https://doi.org/10.1002/cncr.27466
Vance V, Mourtzakis M, McCargar L et al (2011) Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev 12(4):282–294. https://doi.org/10.1111/j.1467-789X.2010.00805.x
Bradshaw PT, Ibrahim JG, Stevens J et al (2012) Postdiagnosis change in bodyweight and survival after breast cancer diagnosis. Epidemiology 23(2):320–327. https://doi.org/10.1097/EDE.0b013e31824596a1
Caan BJ, Kwan ML, Shu XO et al (2012) Weight change and survival after breast cancer in the after breast cancer pooling project. Cancer Epidemiol Biomark Prev 21(8):1260–1271. https://doi.org/10.1158/1055-9965.Epi-12-0306
Norman JE, Bild D, Lewis CE et al (2003) The impact of weight change on cardiovascular disease risk factors in young black and white adults: the CARDIA study. Int J Obes 27(3):369–376. https://doi.org/10.1038/sj.ijo.0802243
Truesdale KP, Stevens J, Lewis CE et al (2006) Changes in risk factors for cardiovascular disease by baseline weight status in young adults who maintain or gain weight over 15 years: the CARDIA study. Int J Obes 30(9):1397–1407. https://doi.org/10.1038/sj.ijo.0803307
Blanchard CM, Courneya KS, Stein K (2008) Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol 26(13):2198–2204. https://doi.org/10.1200/jco.2007.14.6217
Hair BY, Hayes S, Tse CK et al (2014) Racial differences in physical activity among breast cancer survivors. Cancer 120(14):2174–2182. https://doi.org/10.1002/cncr.28630
Jiralerspong S, Kim ES, Dong W et al (2013) Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol 24(10):2506–2514. https://doi.org/10.1093/annonc/mdt224
World Cancer Research Fund/American Institute for Cancer Research (2009) Policy and action for cancer prevention: food, nutrition and physical activity, a global perspective. AICR, Washington, DC, p 4
Weaver KE, Foraker RE, Alfano CM et al (2013) Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Surviv 7(2):253–261. https://doi.org/10.1007/s11764-013-0267-9
Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors [published correction appears in CA Cancer J Clin. 2013 May;63(3):215]. CA Cancer J Clin. 2012;62(4):243–274. https://doi.org/10.3322/caac.21142
Rosti G, Romano F, Secondino S, et al. The role of nutritional support in cured/chronic patients. Nutrients. 2020;12(10). https://doi.org/10.3390/nu12103167.
Ligibel JA, Alfano CM, Courneya KS et al (2014) American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol 32(31):3568–3574. https://doi.org/10.1200/JCO.2014.58.4680
World Health Organization. Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/ July 15 2015.
WHO Consultation on Obesity, Division of Noncommunicable Diseases, Programme of Nutrition, Family and Reproductive Health. Obesity preventing and managing the global epidemic: report of a WHO consultation on obesity, 3–5 June 1997. World Health Organization, Geneva, 1998. [http://apps.who.int/iris/handle/10665/63854]
Włodarczyk M, Nowicka G. Obesity, DNA damage, and development of obesity-related diseases. Int J Mol Sci. 2019;20(5):1146. Published 2019 Mar 6. https://doi.org/10.3390/ijms20051146
Khandekar MJ, Cohen P, Spiegelman BM (2011) Molecular mechanisms of cancer development in obesity. Nat Rev Cancer 11(12):886–895. https://doi.org/10.1038/nrc3174
Goodwin PJ, Ennis M, Pritchard KI et al (2002) Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol 20(1):42–51. https://doi.org/10.1200/jco.20.1.42
Irwin ML, Duggan C, Wang CY et al (2011) Fasting C-peptide levels and death resulting from all causes and breast cancer: the health, eating, activity, and lifestyle study. J Clin Oncol 29(1):47–53. https://doi.org/10.1200/jco.2010.28.4752
World Cancer Research Fund. Be a healthy weight.www.wcrf.org/dietandcancer/recommendations/be-healthy-weight (accessed November 19 2019).
Reeves MM, Terranova CO, Eakin EG et al (2014) Weight loss intervention trials in women with breast cancer: a systematic review. Obes Rev 15(9):749–768. https://doi.org/10.1111/obr.12190
Shaikh H, Bradhurst P, Ma LX, et al. Body weight management in overweight and obese breast cancer survivors. Cochrane Database Syst Rev. 2020;12:CD012110. https://doi.org/10.1002/14651858.
Office of Cancer Survivorship, NCI About cancer survivorship research: survivorship definitions. 2012. Available from: http:// cancercontrol.cancer.gov/ocs/researcher_factsheet.pdf
Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Egger M, Davey Smith G, Schneider M et al (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629
Higgins JP, Thompson SG, Altman DJJ, DG, (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Harvie M, Pegington M, McMullan D et al (2019) The effectiveness of home versus community-based weight control programmes initiated soon after breast cancer diagnosis: a randomised controlled trial. Br J Cancer 121(6):443–454. https://doi.org/10.1038/s41416-019-0522-6
Ferrante JM, Devine KA, Bator A et al (2018) Feasibility and potential efficacy of commercial mHealth/eHealth tools for weight loss in African American breast cancer survivors: pilot randomized controlled trial. Translational behavioral medicine. https://doi.org/10.1093/tbm/iby124
Arikawa AY, Kaufman BC, Raatz SK, et al. Effects of a parallel-arm randomized controlled weight loss pilot study on biological and psychosocial parameters of overweight and obese breast cancer survivors. Pilot and feasibility studies 2018;4(1) https://doi.org/10.1186/s40814-017-0160-9
Stolley M, Sheean P, Gerber B et al (2017) Efficacy of a weight loss intervention for African American breast cancer survivors. J Clin Oncol 35(24):2820–2828. https://doi.org/10.1200/JCO.2016.71.9856
Reeves M, Winkler E, McCarthy N et al (2017) The Living Well after Breast Cancer™ Pilot Trial: a weight loss intervention for women following treatment for breast cancer. Asia Pac J Clin Oncol 13(3):125–136. https://doi.org/10.1111/ajco.12629
Santa-Maria CA, Coughlin JW, Sharma D et al (2020) The effects of a remote based weight loss program on adipocytokines, metabolic markers, and telomere length in breast cancer survivors: the POWER-Remote Trial. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-19-2935
Harrigan M, Cartmel B, Loftfield E et al (2016) Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: the lifestyle, exercise, and nutrition (LEAN) study. J Clin Oncol 34(7):669–676. https://doi.org/10.1200/JCO.2015.61.6375
Greenlee HA, Crew KD, Mata JM et al (2013) A pilot randomized controlled trial of a commercial diet and exercise weight loss program in minority breast cancer survivors. Obesity (silver spring, md) 21(1):65–76. https://doi.org/10.1002/oby.20245
Raji Lahiji M, Najafi S, Janani L, et al. The effect of synbiotic on glycemic profile and sex hormones in overweight and obese breast cancer survivors following a weight-loss diet: a randomized, triple-blind, controlled trial. Clinical nutrition (Edinburgh, Scotland) 2020 https://doi.org/10.1016/j.clnu.2020.05.043
Thomson CA, Stopeck AT, Bea JW, et al. Changes in body weight and metabolic indexes in overweight breast cancer survivors enrolled in a randomized trial of low-fat vs. reduced carbohydrate diets. Nutrition and cancer 2010;62(8):1142‐52. https://doi.org/10.1080/01635581.2010.513803
Anderson C, Harrigan M, George SM et al (2016) Changes in diet quality in a randomized weight loss trial in breast cancer survivors: the lifestyle, exercise, and nutrition (LEAN) study. NPJ Breast Cancer 2:16026. https://doi.org/10.1038/npjbcancer.2016.26
Chan DSM, Vieira AR, Aune D et al (2014) Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 25(10):1901–1914. https://doi.org/10.1093/annonc/mdu042
Runowicz CD, Leach CR, Henry NL et al (2016) American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol 34(6):611–635. https://doi.org/10.1200/jco.2015.64.3809
Protani M, Coory M, Martin JH (2010) Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 123(3):627–635. https://doi.org/10.1007/s10549-010-0990-0
Niraula S, Ocana A, Ennis M, Goodwin PJ (2012) Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat 134(2):769–781. https://doi.org/10.1007/s10549-012-2073-x
Kwan ML, John EM, Caan BJ et al (2014) Obesity and mortality after breast cancer by race/ethnicity: the California Breast Cancer Survivorship Consortium. Am J Epidemiol 179(1):95–111. https://doi.org/10.1093/aje/kwt233
Arias Téllez MJ, Acosta FM, Sanchez-Delgado G, et al. Association of neck circumference with anthropometric indicators and body composition measured by DXA in young Spanish adults. Nutrients. 2020;12(2). https://doi.org/10.3390/nu12020514.
Dunkley AJ, Charles K, Gray LJ et al (2012) Effectiveness of interventions for reducing diabetes and cardiovascular disease risk in people with metabolic syndrome: systematic review and mixed treatment comparison meta-analysis. Diabetes Obes Metab 14(7):616–625. https://doi.org/10.1111/j.1463-1326.2012.01571.x
Wieland LS, Falzon L, Sciamanna CN, et al. Interactive computer-based interventions for weight loss or weight maintenance in overweight or obese people. Cochrane Database Syst Rev. 2012;8(8):Cd007675. https://doi.org/10.1002/14651858.CD007675.pub2.
Physical Activity Guidelines Advisory Committee (2008) Physical Activity Guidelines Advisory Committee report, 2008. US Department of Health and Human Services, Washington, DC
McTiernan, Anne. “Obesity and cancer: the risks, science, and potential management strategies.” Oncology (Williston Park, N.Y.) vol. 19,7 (2005): 871–81; discussion 881–2, 885–6.
Byers T, Sedjo RL (2011) Does intentional weight loss reduce cancer risk? Diabetes Obes Metab 13(12):1063–1072. https://doi.org/10.1111/j.1463-1326.2011.01464.x
Albuquerque KV, Price MR, Badley RA et al (1995) Pre-treatment serum levels of tumour markers in metastatic breast cancer: a prospective assessment of their role in predicting response to therapy and survival. Eur J Surg Oncol 21(5):504–509. https://doi.org/10.1016/s0748-7983(95)96935-7
Al Murri AM, Bartlett JM, Canney PA et al (2006) Evaluation of an inflammation-based prognostic score (GPS) in patients with metastatic breast cancer. Br J Cancer 94(2):227–230. https://doi.org/10.1038/sj.bjc.6602922
Potter GD, Skene DJ, Arendt J et al (2016) Circadian rhythm and sleep disruption: causes, metabolic consequences, and countermeasures. Endocr Rev 37(6):584–608. https://doi.org/10.1210/er.2016-1083
Troesch B, Eggersdorfer M, Laviano A, et al. Expert opinion on benefits of long-chain omega-3 fatty acids (DHA and EPA) in aging and clinical nutrition. Nutrients. 2020;12(9):2555. Published 2020 Aug 24. https://doi.org/10.3390/nu12092555
Kamarajah SK, Bundred J, Tan BHL. Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis [published correction appears in Gastric Cancer. 2019 Mar 1;:]. Gastric Cancer. 2019;22(1):10–22. https://doi.org/10.1007/s10120-018-0882-2
Matsunaga T, Miyata H, Sugimura K et al (2019) Prognostic significance of sarcopenia and systemic inflammatory response in patients with esophageal cancer. Anticancer Res 39(1):449–458. https://doi.org/10.21873/anticanres.13133
Mintziras I, Miligkos M, Wächter S et al (2018) Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: Systematic review and meta-analysis. Int J Surg 59:19–26. https://doi.org/10.1016/j.ijsu.2018.09.014
Guest DD, Evans EM, Rogers LQ (2013) Diet components associated with perceived fatigue in breast cancer survivors. Eur J Cancer Care (Engl) 22(1):51–59. https://doi.org/10.1111/j.1365-2354.2012.01368.x
Knight BB, Oprea-Ilies GM, Nagalingam A et al (2011) Survivin upregulation, dependent on leptin-EGFR-Notch1 axis, is essential for leptin-induced migration of breast carcinoma cells. Endocr Relat Cancer 18(4):413–428. https://doi.org/10.1530/erc-11-0075
Ballard-Barbash R, Friedenreich CM, Courneya KS et al (2012) Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst 104(11):815–840. https://doi.org/10.1093/jnci/djs207
Löf M, Bergström K, Weiderpass E (2012) Physical activity and biomarkers in breast cancer survivors: a systematic review. Maturitas 73(2):134–142. https://doi.org/10.1016/j.maturitas.2012.07.002
Ma C, Avenell A, Bolland M et al (2017) Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ 359:j4849. https://doi.org/10.1136/bmj.j4849
Acknowledgements
Not applicable
Author information
Authors and Affiliations
Contributions
The conception and design of the review: Shurui Wang, Ting Yang, Wanmin Qiang, Zihan Zhao, Aomei Shen, Fangyuan Zhang.
Literature retrieval: Shurui Wang, Ting Yang.
Literature screening: Shurui Wang, Ting Yang, Zihan Zhao.
Quality assessment: Shurui Wang, Ting Yang, Zihan Zhao, Aomei Shen.
Data analysis: Shurui Wang, Wanmin Qiang, Aomei Shen, Fangyuan Zhang.
Writing – original draft: Shurui Wang.
Writing – review and editing: Shurui Wang, Ting Yang, Wanmin Qiang, Zihan Zhao, Aomei Shen, Fangyuan Zhang.
All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. As this is a systematic review reporting on existing data, ethics approval was not required.
Consent for publication
All authors read and approved the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, S., Yang, T., Qiang, W. et al. Benefits of weight loss programs for breast cancer survivors: a systematic reviews and meta-analysis of randomized controlled trials. Support Care Cancer 30, 3745–3760 (2022). https://doi.org/10.1007/s00520-021-06739-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06739-z