Abstract
Background
We recently reported the efficacy of indigo naturalis (IN) in patients with active ulcerative colitis (UC) in a randomized controlled trial (INDIGO study). However, few studies have been conducted to investigate whether IN is effective even in treatment-refractory cases, such as in those with steroid dependency and anti-TNF refractoriness.
Methods
In the INDIGO study, 86 patients with active UC were randomly assigned to an IN group (0.5–2.0 g daily) or placebo group. The rate of clinical response (CR), mucosal healing (MH), and change in fecal calprotectin (FCP) levels was compared between refractory [patients with steroid-dependent disease, previous use of anti-TNF-α, and concomitant use of immunomodulators (IM)] and non-refractory patients. We also analyzed factors predicting CR and MH at week 8.
Results
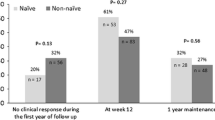
The rates of CR of IN group were significantly higher than placebo group, even in patients with steroid-dependent disease (p < 0.001), previous use of anti-TNF-α (p = 0.002), and concomitant use of IM (p = 0.013). The rates of MH in IN group were significantly higher than in placebo group in patients with steroid-dependent disease (p = 0.009). In the IN group, median FCP levels, at week 8, were significantly lower than baseline in patients with steroid-dependent disease and patients with the previous use of anti-TNF-α (p < 0.001, respectively). Multivariate analysis indicated that the previous use of anti-TNF-α was not a predictive factor for CR and MH at week 8.
Conclusions
In a sub-analysis of data from a randomized placebo-controlled trial, we found that IN may be useful even in patients with steroid-dependent disease and patients with the previous use of anti-TNF-α.



Similar content being viewed by others
Abbreviations
- AE:
-
Adverse event
- AhR:
-
Aryl hydrocarbon receptor
- CRP:
-
C-reactive protein
- CR:
-
Clinical response
- FCP:
-
Fecal calprotectin
- FIT:
-
Fecal immunochemical blood test
- IN:
-
Indigo naturalis
- IQR:
-
Interquartile range
- ITT:
-
Intention-to-treat
- MES:
-
Mayo endoscopic score
- MH:
-
Mucosal healing
- MTWSI:
-
Modified Truelove and Witts Severity Index
- PAH:
-
Pulmonary arterial hypertension
- RCT:
-
Randomized controlled trial
- UC:
-
Ulcerative colitis
References
Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017;389:1756–70.
Arai M, Naganuma M, Sugimoto S, et al. The ulcerative colitis endoscopic index of severity is useful to predict medium- to long-term prognosis in ulcerative colitis patients with clinical remission. J Crohns Colitis. 2016;10:1303–9.
Froslie KF, Jahnsen J, Moum BA, et al. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–22.
Naganuma M, Mizuno S, Nanki K, et al. Recent trends and future directions for the medical treatment of ulcerative colitis. Clin J Gastroenterol. 2016;9:329–36.
Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–85.
Qiu J, Guo X, Chen ZM, et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39:386–99.
Sugimoto S, Naganuma M, Kanai T. Indole compounds may be promising medicines for ulcerative colitis. J Gastroenterol. 2016;51:853–61.
Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357:6349.
Sugimoto S, Naganuma M, Kiyohara H, et al. Clinical efficacy and safety of oral qing-dai in patients with ulcerative colitis: a single-center open-label prospective study. Digestion. 2016;93:193–201.
Lamas B, Richard ML, Leducq V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22:598–605.
Naganuma M, Sugimoto S, Mitsuyama K, et al. Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology. 2018;154:935–47.
Nishio M, Hirooka K, Doi Y. Chinese herbal drug natural indigo may cause pulmonary artery hypertension. Eur Heart J. 2016;37:1992.
Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–9.
Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841–5.
Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965–90.
Nakarai A, Kato J, Hiraoka S, et al. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am J Gastroenterol. 2013;108:83–9.
Gisbert JP, Linares PM, McNicholl AG, et al. Meta-analysis: the efficacy of azathioprine and mercaptopurine in ulcerative colitis. Aliment Pharmacol Ther. 2009;30:126–37.
Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76.
Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–65.
Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85–95.
Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:96–109.
Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710.
Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–36.
Amiot A, Grimaud JC, Peyrin-Biroulet L, et al. Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016;14:1593–601.
Sugimoto K, Ogawa A, Mizoguchi E, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–44.
D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–24.
Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364–8.
Naganuma M, Sugimoto S, Suzuki H, et al. Adverse events in patients with ulcerative colitis treated with indigo naturalis: a Japanese nationwide survey. J Gastroenterol. 2019. https://doi.org/10.1007/s00535-019-01591-9.
Acknowledgements
This study was supported in part by grants from the Keio Gijuku Fukuzawa Memorial Fund for the Advancement of Education and Research (to M. Naganuma). We would like to thank Editage (www.editage.jp) for English language editing.
INDIGO study group: Members in the INDIGO Study Group are Makoto Naganuma, Shinya Sugimoto, Shinta Mizuno, Yoshihiro Nakazato, Tomohiro Fukuda, Toshiaki Teratani, Haruhiko Ogata, Yasushi Iwao, Takanori Kanai (Keio University School of Medicine), Hiroshi Yamasaki, Keiichi Mitsuyama (Kurume University School of Medicine), Taku Kobayashi, Takahiko Toyonaga, Masaru Nakano, Toshifumi Hibi (Kitasato Institute Hospital), Naoki Yoshimura (Yamate Medical Center), Yoichi Sameshima, Hidehisa Ohi (Imamura hospital), Ryohei Hayashi, Yoshitaka Ueno, Shinji Tanaka (Hiroshima University Hospital), Shigeki Bamba, Akira Andoh (Shiga University of Medical Science), Mamoru Watanabe (Tokyo Medical and Dental University), Keiichiro Saigusa, Atsushi Nakazawa (Tokyo Saiseikai Central Hospital), Yuichi Morohoshi, Yuji Koike (Yokohama Municipal Citizen’s Hospital), Jin Imai, Hitoshi Ichikawa (Tokai University Hachioji Hospital), Takahiro Shimoyama, Takayuki Yamamoto (Yokkaichi Hazu Medical Center), Katsuyoshi Matsuoka, Ken Takeuchi, Yasuo Suzuki (Toho University Sakura Medical Center), Mitsuo Nagasaka, Naoki Ohmiya (Fujita Health University School of Medicine), Atsuo Kitano (Wakakusa Daiichi Hospital), Shinya Ashizuka, Haruhiko Inatsu (University of Miyazaki), Kei Onodera, Hiroshi Nakase (Sapporo Medical University School of Medicine), Kazuya Kitamura (Kanazawa University Hospital), Kentaro Ikeya, Hiroyuki Hanai (Hamamatsu South Hospital), Chikako Watanabe, Ryota Hokari (National Defense Medical College), Fumihito Hirai (Fukuoka University Chikushi Hospital), Yuji Naito (Kyoto prefecture University), Namiko Hoshi (Kobe University), Fukunori Kinjo (Urazoe General Hospical), Yo Ishiguro (Hirosaki National Hospital), Makoto Sasaki (Aichi Medical University), Takayuki Matsumoto (Iwate Medical University), Kenji Watanabe (Hyogo College of Medicine), Tadakazu Hisamatsu (Kyorin University School of Medicine), Fumiya Sano, Rachel Roberts, Takayuki Abe (Keio University), Wataru Suda (The University of Tokyo) and Masahira Hattori (Waseda University), Shinji Fukuda, and Akiyoshi Hirayama (Institute for Advanced Biosciences, Keio University).
Funding
This study was funded by Keio Fukuzawa Memorial Fund.
Author information
Authors and Affiliations
Consortia
Contributions
MN and TKa conceived the study. MN designed the main concept of this study. MN, SS, TKo, KM, and KW drafted the main protocol. TA participated in the statistical analysis. All authors participated in patient enrolment and clinical data acquisition. MN and SS drafted and wrote the manuscript. All authors contributed to critical review and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
Makoto Naganuma received commercial research funding from EA Pharma Co., Ltd. and Mochida Pharmaceutical Co., Ltd., outside the submitted work. Taku Kobayashi received advisory fee from Alfresa Pharma, Covidien, Eli Lilly Japan K.K, Ferring Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Thermo Fisher Scientific Inc. and received lecture fees from Mitsubishi Tanabe Pharma Corp., AbbVie GK, Kyorin Pharmaceutical Co., Ltd., Pfizer Japan Inc., Nippon Kayaku Co., Ltd., Takeda Pharmaceutical Co., Ltd. Zeria Pharmaceutical Co., Ltd., Astellas Pharma Inc., Mochida Pharmaceutical Co., Ltd., EA Pharma Co., Ltd., Asahi Kasei Medical Co., Ltd., Alfresa Pharma, Ezai Pharmaceutical Co., Ltd., Gilead, Janssen Pharmaceutical K.K., and received commercial research funding from EA Pharma Co., Ltd., Thermo Fisher Scientific Inc., Alfresa Pharma, Asahi Kasei Medical Co., Ltd. Nippon Kayaku Co., outside the submitted work. Naoki Yoshimura received lecture fees from Mitsubishi Tanabe Pharma Corp., AbbVie GK, and Mochida Pharmaceutical Co., Ltd., outside the submitted work. Naoki Omiya received lecture fees from Mylan EPD and commercial research funding from Mylan EPD, Daiichi Sankyo Co., Ltd, Eli Lilly Japan K.K, Takeda Pharmaceutical Co., Ltd., Bristol-Myers Squibb K.K., outside the submitted work. Yasuo Suzuki received lecture fees from Mitsubishi Tanabe Pharma Corp., Mochida Pharmaceutical Co., Ltd., AbbVie GK, Zeria Pharmaceutical co., Ltd., Kyorin Pharmaceutical Co., Ltd. Janssen Pharmaceutical K.K., Kyorin Pharmaceutical Co., Ltd., EA Pharma Co., Ltd. and research grants from Mitsubishi Tanabe Pharma Corp. and AbbVie GK, EA Pharma Co., Ltd. Kissei Pharma Co., Ltd., JIMRO Co., Ltd., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., outside the submitted work. Tadakazu Hisamatsu received lecture fees from AbbVie GK, EA pharma Co., Ltd., Eisai Co., Ltd., JIMRO Co., Ltd., Mitsubishi Tanabe Pharma Corp., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd. Mochida Pharmaceutical Co., Ltd., and research grants from AbbVie GK, Asahi Kasei Medical Co., Ltd., Astellas Pharma Inc., Daiichi Sankyo Co., Ltd., EA Pharma Co., Ltd., Janssen Pharmaceutical K.K., JIMRO Co., Ltd., Kyorin Pharmaceutical Co., Ltd, Mochida Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., and Zeria Pharmaceutical Co., Ltd., outside the submitted work. Takanori Kanai received lecture fees from Mitsubishi Tanabe Pharma Corp, Astellas Pharma Inc, Miyarisan Pharmaceutical Co., Ltd. AstraZeneca Plc, EA Pharma Co., Ltd., Kyorin Pharmaceutical Co., Ltd., grants from AbbVie GK, Mochida Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corp, Takeda Pharmaceutical Co., Ltd., Nihon Kayaku, Yakult Honsha Co., Ltd., Zeria Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., EA Pharma Co., Ltd., Ezaki Glico Co., Ltd. JIMRO Co., Ltd., EN Otsuka Pharmaceutical Co., Ltd., outside the submitted work. All other authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of the INDIGO study group are listed in acknowledgements section.
Rights and permissions
About this article
Cite this article
Naganuma, M., Sugimoto, S., Fukuda, T. et al. Indigo naturalis is effective even in treatment-refractory patients with ulcerative colitis: a post hoc analysis from the INDIGO study. J Gastroenterol 55, 169–180 (2020). https://doi.org/10.1007/s00535-019-01625-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-019-01625-2