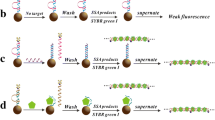
Abstract
We report on an efficient strategy for the preparation of streptavidin (SA)-coated sephadex beads by the reductive amination of the aldehyde groups on the oxidized sephadex beads that were coupled to the free amino groups of SA. The resulting beads have a stable spherical shape with an average diameter of 20–100 μm. The capacity of SA conjugated to 1 mg of these beads is, respectively, ~24 and ~5 times higher than that of commercially available SA-coated polystyrene and sepharose beads. The new beads were successfully applied to the chemiluminescence (CL) detection of telomer DNA via a sandwich-type hybridization assay that involves the following steps: (a) Target DNA is first hybridized with biotinylated capture cDNA immobilized on the SA-coated sephadex beads; (b) the hybrid is then sandwiched with a guanine-rich polymeric probe which (c), is sensitively detected by CL after mixing with a 3,4,5-trimethoxy-phenylglyoxal reagent at room temperature for 10 s. The entire assay can be completed within 2–3 h and has a linear range that extends from 5 to 200 nM concentrations of the target DNA. The lower detection limit is approximately 0.75 nM (1.3 ng · 100 μL−1) in a test tube. Compared to other methods, this one has a wider linear range, a lower detection limit, and is faster. We presume that the new method and materials will be readily applicable to the sensitive detection of target DNA, and also in bead-based CL immunoassays.

We describe a facile strategy for the preparation of streptavidin (SA)-coated sephadex beads with high capacity of SA. The prepared beads were applied to the chemiluminescence detection of a target DNA via a sandwich-type DNA hybridization assay. This assay exhibited high sensitivity and good specificity for the target DNA having a telomere sequence.






Similar content being viewed by others
References
Brenner S, Johnson M, Bridgham J, Golda G, Lloyd DH, Johnson D, Luo S, McCurdy S, Foy M, Ewan M, Roth R, George D, Eletr S, Albrecht G, Vermaas E, Williams SR, Moon K, Burcham T, Pallas M, DuBridge RB, Kirchner J, Fearon K, Mao J, Corcoran K (2000) Gene expression analysis by massively parallel signature sequencing (MPSS) on microbead arrays. Nat Biotechnol 18:630–634
Trau M, Battersby BJ (2001) Novel colloidal materials for high-throughput screening applications in drug discovery and genomics. Adv Mater 13:975–979
Nolan JP, Mandy FF (2001) Suspension array technology: new tools for gene and protein analysis. Cell Mol Biol (Noisy-le-grand) 47:1241–1256
Jani IV, Janossy G, Brown DW, Mandy F (2002) Multiplexed immunoassays by flow cytometry for diagnosis and surveillance of infectious diseases in resource-poor settings. Lancet Infect Dis 2:243–250
Ugozzoli LA (2004) Multiplex assays with fluorescent microbead readout: a powerful tool for mutation detection. Clin Chem 50:1963–1965
Braeckmans K, De Smedt SC, Leblans M, Pauwels R, Demeester J (2002) Encoding microcarriers: present and future technologies. Nat Rev Drug Discov 1:447–456
Fulton RJ, McDade RL, Smith PL, Kienker LJ, Kettman JR (1997) Advanced multiplexed analysis with the FlowMetrixTM system. J Clin Chem 43:1749–1756
Elshal MF, McCoy JP (2006) Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods 38:317–323
Kellar KL, Iannone MA (2002) Multiplexed micro sphere based flow cytometric assays. Exp Hematol 30:1227–1237
Nolan JP, Sklar LA (2002) Suspension array technology: evolution of the flat-array paradigm. Trends Biotechnol 20:9–12
Wilson R, Cossins AR, Spiller DG (2006) Encoded microcarriers for high-throughput multiplexed detection. Angew Chem Int Ed 45:6104–6117
Bayer EA, Ben-Hur H, Wickchek M (1990) Isolation and properties of streptavidin. Methods Enzymol 184:80–89
Huhtinen P, Soukka T, Lovgren T (2004) Immunoassay of total prostate-specific antigen using europium (III) nanoparticle labels and streptavidin–biotin technology. J Immunol Methods 294:111–122
Wilchek M, Bayer EA (1998) The avidin-biotin complex in bioanalytical applications. Anal Biochem 171:1–32
Xie X, Zhang X, Gao HF (2004) DNA purification and gene typing: based on multifunctional nanobeads. Chin Sci Bull 49:886–889
Sun YR, Wang YQ (2000) Biotin-avidin system and its application. HighTech Lett 10:95–97
Valimaa L, Pettersson K, Rosenberg J (2004) Quantification of streptavidin adsorption in microtitration wells. Anal Biochem 331:376–384
Davies J, Dawkes AC, Haymes AG (1994) A scanning tunneling microscopy comparison of passive antibody adsorption and biotinylated antibody linkage to streptavidin on microtiter wells. J Immunol Methods 167:263–269
Butler JE (2000) Solid supports in enzyme-linked immunosorbent assay and other solid-phase immunoassays. Methods 22:4–23
Peterman JH, Tarcha PJ, Chu VP, Butler JE (1998) The immunochemistry of sandwich-ELISAs IV. the antigen capture capacity of antibody covalently attached to bromoacetyl surface-functionalized polystyrene. J Immunol Methods 111:271–275
Hermanson GT, Mallia AK, Smith PK (1992) Immobilized Affinity Ligand Techniques. Academic, New York
Dudin G, Steegmayer EW, Vogt P, Schnitzer H, Diaz E, Howell KE, Cremer T, Cremer C (1988) Sorting of chromosomes by magnetic separation. Hum Genet 80:111116
Zhang Z, Zhu H, Tang Y, Cui T, Geng T, Chen C, Cui Y (2007) Preparation and application of streptavidin magnetic particles. Sci China Ser B Chem 50:127–134
Berenson RJ, Bensinger WI, Kalamasz D, Schuening F, Deeg HJ, Graham T, Storb R (1987) Engraftment of dogs with Ia-positive marrow cells isolated by avidin- biotin immunoadsorption. Blood 69:1363–1367
Yamasuij M, Shibata T, Kabashima T, Kai M (2011) Chemiluminescence detection of telomere DNA in human cells on a membrane by using fluorescein-5-isothiocyanate-labeled primers. Anal Biochem 413:50–54
EL-Mahdy AFM, Shibata T, Kabashima T, Kai M (2014) Dendrimer-like polymeric DNAs as chemiluminescence probes for amplified detection of telomere DNA on a solid-phase membrane. Chem Commun 50:859–891
Kai M, Kishida S, Sakai K (1999) A chemiluminescence derivatization method for detecting nucleic acids and DNA probes using a trimethoxyphenylglyoxal reagent that recognizes guanine. Anal Chim Acta 381:155–163
Sun CF, Wu ZL, Jia FJ, Wang YF, Li TP, Zhao MJ (2008) Identification of zebrafish LPTS: a gene with similarities to human LPTS/PinX1 that inhibits telomerase activity. Gene 420:90–98
Greider CW, Blackburn EH (1985) Identification of a specific telomere terminal transferase activity in tetrahymena extracts. Cell 43:405–413
Zheng Y, Hu N, Sun Q, Wang C, Taylor PR (2009) Telomere attrition in cancer cells and telomere length in tumor stroma cells predict chromosome instability in esophageal squamous cell carcinoma: a genome-wide analysis. Cancer Res 69:1604–1614
Yingying Q, Li L, Baoxin L (2009) Label-free detection of specific DNA sequence-telomere using unmodified gold nanoparticles as colorimetric probes. Spectrochim Acta A 74:127–131
Li T, Dong S, Wang E (2007) Enhanced catalytic DNAzyme for label-free colorimetric detection of DNA. Chem Commun 41:4209–4211
Xin-Chun G, Jun L, Hao-Li Z (2010) Monitoring human telomere DNA hybridization and G-quadruplex formation using gold nanorods. Anal Chim Acta 668:208–214
Richard MC (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 30:10
Xiao Y, Qu X, Plaxco KW, Heeger AJ (2007) Label-free electrochemical detection of DNA in blood serum via target-induced resolution of an electrode-bound DNA pseudoknot. J Am Chem Soc 129:11896–11897
Kerman K, Vestergarad MDL, Nagataru N, Takamura Y, Tamiya E (2006) Electrochemical genosensor based on peptide nucleic acid-mediated PCR and asymmetric PCR techniques: electrostatic interactions with a metal cation. Anal Chem 78:2182–2189
Nelson BP, Grimsrud TE, Liles MR, Goodman RM, Corn RM (2001) Surface plasmon resonance imaging measurements of DNA and RNA hybridization adsorption onto DNA microarrays. Anal Chem 73:1–7
Lu J, Lau C, Kai M (2003) Magnetic bead-based label-free chemilumines-cence detection of telomeres. Chem Commun 23:2888–2889
Pavlov V, Xiao Y, Gill R, Dishon A, Kotler M, Wilner I (2004) Amplified chemiluminescence surface detection of DNA and telomerase activity using catalytic nucleic acid labels. Anal Chem 76:2152–2156
Micromod Partikeltechnologie GmbH, Germany, http://www.micromod.de/pdf/aktuell/01-19-503_tds_en.pdf
Invitrogen, USA, http://tools.lifetechnologies.com/content/sfs/manuals/434341_Rev0609.pdf
Peng Q, Cao Z, Lau C, Kai M, Lu J (2011) Aptamer-barcode based immunoassay for the instantaneous derivatization chemiluminescence detection of IgE coupled to magnetic beads. Analyst 136:140–147
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and also supported partially by the Global Center of Excellence Program at Nagasaki University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Mahdy, A.F.M., Ejupi, V., Shibata, T. et al. Facile preparation of streptavidin-coated sephadex beads and their application to chemiluminescence detection of a target DNA. Microchim Acta 182, 495–503 (2015). https://doi.org/10.1007/s00604-014-1348-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1348-9