Abstract
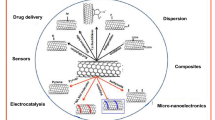
This review (with 142 references) summarize the state of the art in molecularly imprinting technology as applied to the surface of carbon nanotubes (CNTs) which result in so-called CNTs@MIPs. These nanomaterials offer a remedy to the flaws of traditional MIPs, such as poor site accessibility for templates, slow mass transfer and template leakage. They also are flexible in that different materials can be integrated with CNTs. Given the advantages of using CNT@MIPs, this technology has experienced rapid expansion, not the least because CNT@MIPs can be produced at low cost and by a variety of synthetic approaches. We summarize methods of, and recent advances in the synthesis of CNT@MIPs, and then highlight some representative applications. We also comment on their potential future developments and research directions.

ᅟ








Similar content being viewed by others
Abbreviations
- 2 or 4-VP:
-
2-or 4-vinylpyridine
- AA:
-
Acrylic acid
- AEP:
-
(p-acryloylaminophenyl)-{(4-aminophenyl)-diethyl ammonium}-ethylphosphate
- AIBN:
-
Azo-bisisobutyronitrile
- APS:
-
Ammonium persulfate
- APTMS:
-
Aminopropyltrimethoxysilane
- β-CD:
-
Beta-cyclodextrin
- BDC:
-
N,N-diethyldithiocarbamate
- BSA:
-
Bovine serum albumin
- CLRP:
-
Controlled/living free radical polymerization
- CNTs:
-
Carbon nanotubes
- CNTs@MIPs:
-
Molecularly imprinted polymers on the surface of carbon nanotubes
- CS:
-
Chitosan
- CSDT:
-
Chitosan derivative
- DMAc:
-
Dimethyl acetamide
- DMF:
-
Dimethyl formamide
- DMSO:
-
Dimethyl sulfoxide
- DVB:
-
Divinylbenzene
- ECL:
-
Electrochemiluminescence
- EE:
-
Ethoxyethanol
- EGDMA or EDMA:
-
Ethylene glycol dimethacrylate
- FQs:
-
Fluoroquinolones
- Ga(III):
-
Gallium(III)
- MAA:
-
Methacrylic acid
- MIM:
-
Molecularly imprinted membrane
- MIPPy:
-
Molecular imprinted polypyrrole
- MIPs:
-
Molecularly imprinted polymers
- MIT:
-
Molecular imprinting technology
- MTMOS:
-
Methyltrimethoxysilane
- MWNTs:
-
Multiwalled carbon nanotubes
- NNMBA:
-
N,N-methylenebisacrylamide
- NPA:
-
4-nitrophenyl acrylate
- OTA:
-
Ochratoxin A
- PAMAM:
-
Polyamide amine
- PEG:
-
Polyethylene glycol
- PETRA:
-
Pentaerythritol triacrylate
- PPy:
-
Polypyrrole
- PTMOS:
-
phenyltrimethoxysilane
- SPE:
-
Solid-phase extraction
- SPME:
-
Solid phase micro extraction
- SWNTs:
-
Single-walled carbon nanotubes
- TCP:
-
2,4,6-Trichlorophenol
- TEOS:
-
Tetraethylorthosilicate
- THF:
-
Tetrahydrofuran
- μ-SPP:
-
Micro-solid phase preconcentration
References
Hinterdorfer P, Dufrene YF (2006) Detection and localization of single molecular recognition events using atomic force microscopy. Nat Methods 3(5):347–355. doi:10.1038/nmeth871
Ye L, Mosbach K (2008) Molecular imprinting: synthetic materials as substitutes for biological antibodies and receptors†. Chem Mater 20(3):859–868. doi:10.1021/cm703190w
Wulff G, Sarhan A (1972) Über die Anwendung von enzymanalog gebauten Polymeren zur Racemattrennung. Angew Chem 84(8):364. doi:10.1002/ange.19720840838
Vlatakis G, Andersson LI, Muller R, Mosbach K (1993) Drug assay using antibody mimics made by molecular imprinting. Nature 361(6413):645–647. doi:10.1038/361645a0
Arshady R, Mosbach K (1981) Synthesis of substrate-selective polymers by host-guest polymerization. Die Makromol Chem 182(2):687–692. doi:10.1002/macp.1981.021820240
Nicholls IA, Rosengren JP (2001) Molecular imprinting of surfaces. Bioseparation 10(6):301–305. doi:10.1023/a:1021541631063
Wulff G (2002) Enzyme-like catalysis by molecularly imprinted polymers. Chem Rev 102(1):1–28. doi:10.1021/cr980039a
Alexander C, Andersson HS, Andersson LI, Ansell RJ, Kirsch N, Nicholls IA, O’Mahony J, Whitcombe MJ (2006) Molecular imprinting science and technology: a survey of the literature for the years up to and including 2003. J Mol Recognit 19(2):106–180. doi:10.1002/jmr.760
Chen L, Xu S, Li J (2011) Recent advances in molecular imprinting technology: current status, challenges and highlighted applications. Chem Soc Rev 40(5):2922–2942. doi:10.1039/c0cs00084a
Díaz-Díaz G, Antuña-Jiménez D, Carmen Blanco-López M, Jesús Lobo-Castañón M, Miranda-Ordieres AJ, Tuñón-Blanco P (2012) New materials for analytical biomimetic assays based on affinity and catalytic receptors prepared by molecular imprinting. TrAC Trends Anal Chem 33:68–80. doi:10.1016/j.trac.2011.09.011
Tan CJ, Tong YW (2007) Molecularly imprinted beads by surface imprinting. Anal Bioanal Chem 389(2):369–376. doi:10.1007/s00216-007-1362-4
Ge Y, Turner AP (2008) Too large to fit? Recent developments in macromolecular imprinting. Trends Biotechnol 26(4):218–224. doi:10.1016/j.tibtech.2008.01.001
Wang H, Zhao H, Quan X, Chen S (2011) Electrochemical determination of tetracycline using molecularly imprinted polymer modified carbon nanotube-gold nanoparticles electrode. Electroanalysis 23(8):1863–1869. doi:10.1002/elan.201100049
Zhang Z, Yang X, Chen X, Zhang M, Luo L, Peng M, Yao S (2011) Novel magnetic bovine serum albumin imprinted polymers with a matrix of carbon nanotubes, and their application to protein separation. Anal Bioanal Chem 401(9):2855–2863. doi:10.1007/s00216-011-5373-9
Nagata T, Goji S, Akamatsu K, Nawafune H, Matsui J (2012) Monodispersed molecularly imprinted polymer beads with enhanced atrazine retention ability synthesized with polymeric diluents. Anal Lett 45(9):977–985. doi:10.1080/00032719.2012.670786
Fan L, Zhang Y, Li X, Luo C, Lu F, Qiu H (2012) Removal of alizarin red from water environment using magnetic chitosan with Alizarin Red as imprinted molecules. Colloids Surf, B 91:250–257. doi:10.1016/j.colsurfb.2011.11.014
Xia YQ, Guo TY, Song MD, Zhang BH, Zhang BL (2006) Selective separation of quercetin by molecular imprinting using chitosan beads as functional matrix. React Funct Polym 66(12):1734–1740. doi:10.1016/j.reactfunctpolym.2006.08.001
Song X, Li C, Xu R, Wang K (2012) Molecular-ion-imprinted chitosan hydrogels for the selective adsorption of silver(I) in aqueous solution. Ind Eng Chem Res 51(34):11261–11265. doi:10.1021/ie3010989
Dai C, Liu C, Wei J, Hong H, Zhao Q (2010) Molecular imprinted macroporous chitosan coated mesoporous silica xerogels for hemorrhage control. Biomaterials 31(30):7620–7630. doi:10.1016/j.biomaterials.2010.06.049
Gu JY, Zhang H, Yuan G, Chen LR, Xu XJ (2011) Surface-initiated molecularly imprinted polymeric column: in situ synthesis and application for semi-preparative separation by high performance liquid chromatography. J Chromatogr A 1218(45):8150–8155. doi:10.1016/j.chroma.2011.09.019
Zhao D, Jia J, Yu X, Sun X (2011) Preparation and characterization of a molecularly imprinted polymer by grafting on silica supports: a selective sorbent for patulin toxin. Anal Bioanal Chem 401(7):2259–2273. doi:10.1007/s00216-011-5282-y
Zhang W, Qin L, He XW, Li WY, Zhang YK (2009) Novel surface modified molecularly imprinted polymer using acryloyl-beta-cyclodextrin and acrylamide as monomers for selective recognition of lysozyme in aqueous solution. J Chromatogr A 1216(21):4560–4567. doi:10.1016/j.chroma.2009.03.056
Xia YQ, Guo TY, Zhao HL, Song MD, Zhang BH, Zhang BL (2009) Protein recognition onto silica particles using chitosan as intermedium substrate. J Biomed Mater Res, Part A 90A(2):326–332. doi:10.1002/jbm.a.32084
Moreira FTC, Dutra RAF, Noronha JPC, Sales MGF (2011) Myoglobin-biomimetic electroactive materials made by surface molecular imprinting on silica beads and their use as ionophores in polymeric membranes for potentiometric transduction. Biosens Bioelectron 26(12):4760–4766. doi:10.1016/j.bios.2011.05.045
Hua Z, Zhou S, Zhao M (2009) Fabrication of a surface imprinted hydrogel shell over silica microspheres using bovine serum albumin as a model protein template. Biosens Bioelectron 25(3):615–622. doi:10.1016/j.bios.2009.01.027
Fukazawa K, Li Q, Seeger S, Ishihara K (2013) Direct observation of selective protein capturing on molecular imprinting substrates. Biosens Bioelectron 40(1):96–101. doi:10.1016/j.bios.2012.06.033
Fukazawa K, Ishihara K (2009) Fabrication of a cell-adhesive protein imprinting surface with an artificial cell membrane structure for cell capturing. Biosens Bioelectron 25(3):609–614. doi:10.1016/j.bios.2009.02.034
Chang L, Li Y, Chu J, Qi J, Li X (2010) Preparation of core-shell molecularly imprinted polymer via the combination of reversible addition-fragmentation chain transfer polymerization and click reaction. Anal Chim Acta 680(1–2):65–71. doi:10.1016/j.aca.2010.09.017
Barahona F, Turiel E, Cormack PAG, Martin-Esteban A (2010) Chromatographic performance of molecularly imprinted polymers: core-shell microspheres by precipitation polymerization and grafted MIP films via iniferter-modified silica beads. J Polym Sci, Part A: Polym Chem 48(5):1058–1066. doi:10.1002/pola.23860
Dramou P, Xiao D, He H, Liu T, Zou W (2013) Loading behavior of gatifloxacin in urine and lake water on a novel magnetic molecularly imprinted polymer used as extraction sorbent with spectrophotometric analysis. J Sep Sci 36(5):898–906. doi:10.1002/jssc.201200831
Wen T, Xue C, Li Y, Wang Y, Wang R, Hong J, Zhou X, Jiang H (2012) Reduced graphene oxide-platinum nanoparticles composites based imprinting sensor for sensitively electrochemical analysis of 17 beta-estradiol. J Electroanal Chem 682:121–127. doi:10.1016/j.jelechem.2012.07.015
Mao Y, Bao Y, Gan S, Li F, Niu L (2011) Electrochemical sensor for dopamine based on a novel graphene-molecular imprinted polymers composite recognition element. Biosens Bioelectron 28(1):291–297. doi:10.1016/j.bios.2011.07.034
Liu Y, Zhu L, Zhang Y, Tang H (2012) Electrochemical sensoring of 2,4-dinitrophenol by using composites of graphene oxide with surface molecular imprinted polymer. Sens Actuators, B 171:1151–1158. doi:10.1016/j.snb.2012.06.054
Chang L, Wu S, Chen S, Li X (2011) Preparation of graphene oxide-molecularly imprinted polymer composites via atom transfer radical polymerization. J Mater Sci 46(7):2024–2029. doi:10.1007/s10853-010-5033-z
Pan J, Hang H, Dai X, Dai J, Huo P, Yan Y (2012) Switched recognition and release ability of temperature responsive molecularly imprinted polymers based on magnetic halloysite nanotubes. J Mater Chem 22(33):17167–17175. doi:10.1039/C2jm32821f
Pan J, Wang B, Dai J, Dai X, Hang H, Ou H, Yan Y (2012) Selective recognition of 2,4,5-trichlorophenol by temperature responsive and magnetic molecularly imprinted polymers based on halloysite nanotubes. J Mater Chem 22(8):3360–3369. doi:10.1039/C1jm14825g
Pan J, Yao H, Xu L, Ou H, Huo P, Li X, Yan Y (2011) Selective recognition of 2,4,6-trichlorophenol by molecularly imprinted polymers based on magnetic halloysite nanotubes composites. J Phys Chem C 115(13):5440–5449. doi:10.1021/jp111120x
Zhang W, He XW, Chen Y, Li WY, Zhang YK (2012) Molecularly imprinted polymer anchored on the surface of denatured bovine serum albumin modified CdTe quantum dots as fluorescent artificial receptor for recognition of target protein. Biosens Bioelectron 31(1):84–89. doi:10.1016/j.bios.2011.09.042
Zhang W, He XW, Chen Y, Li WY, Zhang YK (2011) Composite of CdTe quantum dots and molecularly imprinted polymer as a sensing material for cytochrome c. Biosens Bioelectron 26(5):2553–2558. doi:10.1016/j.bios.2010.11.004
Wang HF, He Y, Ji TR, Yan XP (2009) Surface molecular imprinting on Mn-Doped ZnS quantum dots for room-temperature phosphorescence optosensing of pentachlorophenol in water. Anal Chem 81(4):1615–1621. doi:10.1021/ac802375a
Mao Y, Bao Y, Han D, Li F, Niu L (2012) Efficient one-pot synthesis of molecularly imprinted silica nanospheres embedded carbon dots for fluorescent dopamine optosensing. Biosens Bioelectron 38(1):55–60. doi:10.1016/j.bios.2012.04.043
Liu J, Chen H, Lin Z, Lin JM (2010) Preparation of surface imprinting polymer capped Mn-Doped ZnS quantum dots and their application for chemiluminescence detection of 4-nitrophenol in tap water. Anal Chem 82(17):7380–7386. doi:10.1021/ac101510b
Lin HY, Ho MS, Lee MH (2009) Instant formation of molecularly imprinted poly(ethylene-co-vinyl alcohol)/quantum dot composite nanoparticles and their use in one-pot urinalysis. Biosens Bioelectron 25(3):579–586. doi:10.1016/j.bios.2009.03.039
Lian HT, Liu B, Chen YP, Sun XY (2012) A urea electrochemical sensor based on molecularly imprinted chitosan film doping with CdS quantum dots. Anal Biochem 426(1):40–46. doi:10.1016/j.ab.2012.03.024
Ge S, Zhang C, Yu F, Yan M, Yu J (2011) Layer-by-layer self-assembly CdTe quantum dots and molecularly imprinted polymers modified chemiluminescence sensor for deltamethrin detection. Sens Actuators, B 156(1):222–227. doi:10.1016/j.snb.2011.04.024
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354(6348):56–58. doi:10.1038/354056a0
Hirsch A (2002) Functionalization of single-walled carbon nanotubes. Angew Chem Int Ed Engl 41(11):1853–1859. doi:10.1002/1521-3773(20020603)41:11<1853::AID-ANIE1853>3.0.CO;2-N
Sun YP, Fu K, Lin Y, Huang W (2002) Functionalized carbon nanotubes: properties and applications. Acc Chem Res 35(12):1096–1104. doi:10.1021/ar010160v
Dai HJ (2002) Carbon nanotubes: synthesis, integration, and properties. Acc Chem Res 35(12):1035–1044. doi:10.1021/ar0101640
Kim B, Lee Y-H, Ryu J-H, Suh K-D (2006) Enhanced colloidal properties of single-wall carbon nanotubes in α-terpineol and Texanol. Colloids Surf, A 273(1–3):161–164. doi:10.1016/j.colsurfa.2005.08.024
Vaisman L, Wagner HD, Marom G (2006) The role of surfactants in dispersion of carbon nanotubes. Adv Colloid Interface Sci 128–130:37–46. doi:10.1016/j.cis.2006.11.007
Bianco A, Kostarelos K, Prato M (2011) Making carbon nanotubes biocompatible and biodegradable. Chem Commun 47(37):10182–10188. doi:10.1039/c1cc13011k
Singh P, Campidelli S, Giordani S, Bonifazi D, Bianco A, Prato M (2009) Organic functionalisation and characterisation of single-walled carbon nanotubes. Chem Soc Rev 38(8):2214–2230. doi:10.1039/b518111a
Liu J, Rinzler AG, Dai H, Hafner JH, Bradley RK, Boul PJ, Lu A, Iverson T, Shelimov K, Huffman CB, Rodriguez-Macias F, Shon YS, Lee TR, Colbert DT, Smalley RE (1998) Fullerene pipes. Science 280(5367):1253–1256. doi:10.1126/science.280.5367.1253
Frank S, Poncharal P, Wang ZL, Heer WA (1998) Carbon nanotube quantum resistors. Science 280(5370):1744–1746. doi:10.1126/science.280.5370.1744
Raffaelle RP, Landi BJ, Harris JD, Bailey SG, Hepp AF (2005) Carbon nanotubes for power applications. Mater Sci Eng B 116(3):233–243. doi:10.1016/j.mseb.2004.09.034
Li LL, Lin R, He H, Jiang L, Gao MM (2013) Interaction of carboxylated single-walled carbon nanotubes with bovine serum albumin. Spectrochim Acta, Part A 105:45–51. doi:10.1016/j.saa.2012.11.111
Jiang L, Liu TB, He H, Pham-Huy LA, Li LL, Pham-Huy C, Xiao DL (2012) Adsorption behavior of pazufloxacin mesilate on amino-functionalized carbon nanotubes. J Nanosci Nanotechnol 12(9):7271–7279. doi:10.1166/jnn.2012.6562
Chen Z, Pierre D, He H, Tan SH, Chuong PH, Hong H, Huang JL (2011) Adsorption behavior of epirubicin hydrochloride on carboxylated carbon nanotubes. Int J Pharm 405(1–2):153–161. doi:10.1016/j.ijpharm.2010.11.034
Xiao DL, Dramou P, He H, Lien APH, Li H, Yao YY, Chuong PH (2012) Magnetic carbon nanotubes: synthesis by a simple solvothermal process and application in magnetic targeted drug delivery system. J Nanopart Res 14(7):984–996. doi:10.1007/s11051-012-0984-4
Huang J, Xing X, Zhang X, He X, Lin Q, Lian W, Zhu H (2011) A molecularly imprinted electrochemical sensor based on multiwalled carbon nanotube-gold nanoparticle composites and chitosan for the detection of tyramine. Food Res Int 44(1):276–281. doi:10.1016/j.foodres.2010.10.020
Blomgren A, Berggren C, Holmberg A, Larsson F, Sellergren B, Ensing K (2002) Extraction of clenbuterol from calf urine using a molecularly imprinted polymer followed by quantitation by high-performance liquid chromatography with UV detection. J Chromatogr A 975(1):157–164. doi:10.1016/s0021-9673(02)01359-6
Kootstra PR, Kuijpers CJPF, Wubs KL, van Doorn D, Sterk SS, van Ginkel LA, Stephany RW (2005) The analysis of beta-agonists in bovine muscle using molecular imprinted polymers with ion trap LCMS screening. Anal Chim Acta 529(1–2):75–81. doi:10.1016/j.aca.2004.09.053
Xia Y, McGuffey JE, Bhattacharyya S, Sellergren B, Yilmaz E, Wang L, Bernert JT (2005) Analysis of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in urine by extraction on a molecularly imprinted polymer column and liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Anal Chem 77(23):7639–7645. doi:10.1021/ac058027u
Yan H, Cheng X, Yang G (2012) Dummy molecularly imprinted solid-phase extraction for selective determination of five phthalate esters in plastic bottled functional beverages. J Agric Food Chem 60(22):5524–5531. doi:10.1021/jf300660m
Yin YM, Chen YP, Wang XF, Liu Y, Liu HL, Xie MX (2012) Dummy molecularly imprinted polymers on silica particles for selective solid-phase extraction of tetrabromobisphenol A from water samples. J Chromatogr A 1220:7–13. doi:10.1016/j.chroma.2011.11.065
Nemoto K, Kubo T, Nomachi M, Sano T, Matsumoto T, Hosoya K, Hattori T, Kaya K (2007) Simple and effective 3D recognition of domoic acid using a molecularly imprinted polymer. J Am Chem Soc 129(44):13626–13632. doi:10.1021/ja0741426
Tominaga Y, Kubo T, Kaya K, Hosoya K (2009) Effective recognition on the surface of a polymer prepared by molecular imprinting using ionic complex. Macromolecules 42(8):2911–2915. doi:10.1021/ma802880z
Prasad BB, Madhuri R, Tiwari MP, Sharma PS (2010) Imprinting molecular recognition sites on multiwalled carbon nanotubes surface for electrochemical detection of insulin in real samples. Electrochim Acta 55(28):9146–9156. doi:10.1016/j.electacta.2010.09.008
Prasad BB, Prasad A, Tiwari MP (2013) Highly selective and sensitive analysis of gamma-aminobutyric acid using a new molecularly imprinted polymer modified at the surface of abrasively immobilized multi-walled carbon nanotubes on pencil graphite electrode. Electrochim Acta 102:400–408. doi:10.1016/j.electacta.2013.04.043
Courtois J, Fischer G, Schauff S, Albert K, Irgum K (2005) Interactions of bupivacaine with a molecularly imprinted polymer in a monolithic format studied by NMR. Anal Chem 78(2):580–584. doi:10.1021/ac0515733
Whitcombe MJ, Chianella I, Larcombe L, Piletsky SA, Noble J, Porter R, Horgan A (2011) The rational development of molecularly imprinted polymer-based sensors for protein detection. Chem Soc Rev 40(3):1547–1571. doi:10.1039/c0cs00049c
Gholivand MB, Khodadadian M (2011) Rationally designed molecularly imprinted polymers for selective extraction of methocarbamol from human plasma. Talanta 85(3):1680–1688. doi:10.1016/j.talanta.2011.06.066
Baggiani C, Giovannoli C, Anfossi L, Passini C, Baravalle P, Giraudi G (2012) A connection between the binding properties of imprinted and nonimprinted polymers: a change of perspective in molecular imprinting. J Am Chem Soc 134(3):1513–1518. doi:10.1021/ja205632t
Pichon V, Haupt K (2006) Affinity separations on molecularly imprinted polymers with special emphasis on solid-phase extraction. J Liq Chromatogr Relat Technol 29(7–8):989–1023. doi:10.1080/10826070600574739
Michailof C, Manesiotis P, Panayiotou C (2008) Synthesis of caffeic acid and p-hydroxybenzoic acid molecularly imprinted polymers and their application for the selective extraction of polyphenols from olive mill waste waters. J Chromatogr A 1182(1):25–33. doi:10.1016/j.chroma.2008.01.001
Cai W, Gupta RB (2004) Molecularly-imprinted polymers selective for tetracycline binding. Sep Purif Technol 35(3):215–221. doi:10.1016/S1383-5866(03)00143-6
Pardeshi S, Dhodapkar R, Kumar A (2013) Quantum chemical density functional theory studies on the molecular structure and vibrational spectra of Gallic acid imprinted polymers. Spectrochim Acta A Mol Biomol Spectrosc 116:562–573. doi:10.1016/j.saa.2013.07.067
Panahi R, Vasheghani-Farahani E, Shojaosadati SA (2007) Separation of l-lysine from dilute aqueous solution using molecular imprinting technique. Biochem Eng J 35(3):352–356. doi:10.1016/j.bej.2007.01.027
Gao R, Kong X, Su F, He X, Chen L, Zhang Y (2010) Synthesis and evaluation of molecularly imprinted core-shell carbon nanotubes for the determination of triclosan in environmental water samples. J Chromatogr A 1217(52):8095–8102. doi:10.1016/j.chroma.2010.10.121
Zhang Z, Yang X, Zhang H, Zhang M, Luo L, Hu Y, Yao S (2011) Novel molecularly imprinted polymers based on multi-walled carbon nanotubes with binary functional monomer for the solid-phase extraction of erythromycin from chicken muscle. J Chromatogr B Anal Technol Biomed Life Sci 879(19):1617–1624. doi:10.1016/j.jchromb.2011.03.054
Hu YF, Zhang ZH, Zhang HB, Luo LJ, Yao SZ (2012) Sensitive and selective imprinted electrochemical sensor for p-nitrophenol based on ZnO nanoparticles/carbon nanotubes doped chitosan film. Thin Solid Films 520(16):5314–5321. doi:10.1016/j.tsf.2011.11.083
Yu JCC, Lai EPC (2006) Molecularly imprinted polypyrrole modified carbon nanotubes on stainless steel frit for selective micro solid phase pre-concentration of ochratoxin A. React Funct Polym 66(7):702–711. doi:10.1016/j.reactfunctpolym.2005.10.021
Kan X, Zhao Y, Geng Z, Wang Z, Zhu JJ (2008) Composites of multiwalled carbon nanotubes and molecularly imprinted polymers for dopamine recognition. J Phys Chem C 112(13):4849–4854. doi:10.1021/jp077445v
Choong CL, Bendall JS, Milne WI (2009) Carbon nanotube array: a new MIP platform. Biosens Bioelectron 25(3):652–656. doi:10.1016/j.bios.2008.11.025
Chen HJ, Zhang ZH, Luo LJ, Yao SZ (2012) Surface-imprinted chitosan-coated magnetic nanoparticles modified multi-walled carbon nanotubes biosensor for detection of bovine serum albumin. Sens Actuators, B 163(1):76–83. doi:10.1016/j.snb.2012.01.010
Xiao D, Dramou P, Xiong N, He H, Li H, Yuan D, Dai H (2013) Development of novel molecularly imprinted magnetic solid-phase extraction materials based on magnetic carbon nanotubes and their application for the determination of gatifloxacin in serum samples coupled with high performance liquid chromatography. J Chromatogr A 1274:44–53. doi:10.1016/j.chroma.2012.12.011
Liu X, Wang X, Tan F, Zhao H, Quan X, Chen J, Li L (2012) An electrochemically enhanced solid-phase microextraction approach based on molecularly imprinted polypyrrole/multi-walled carbon nanotubes composite coating for selective extraction of fluoroquinolones in aqueous samples. Anal Chim Acta 727:26–33. doi:10.1016/j.aca.2012.03.054
Tan F, Deng M, Liu X, Zhao H, Li X, Quan X, Chen J (2011) Evaluation of a novel microextraction technique for aqueous samples: porous membrane envelope filled with multiwalled carbon nanotubes coated with molecularly imprinted polymer. J Sep Sci 34(6):707–715. doi:10.1002/jssc.201000791
Yen CP, Chin NP, Wei HC, Chuan HK (2010) Detection of uric acid based on multi-walled carbon nanotubes polymerized with a layer of molecularly imprinted PMAA. Sens Actuators, B 146(2):466–471. doi:10.1016/j.snb.2009.11.035
Diaz-Diaz G, Carmen Blanco-Lopez M, Jesus Lobo-Castanon M, Miranda-Ordieres AJ, Tunon-Blanco P (2011) Preparation and characterization of a molecularly imprinted microgel for electrochemical sensing of 2,4,6-trichlorophenol. Electroanalysis 23(1):201–208. doi:10.1002/elan.201000481
Yufang H, Zhaohui Z, Huabin Z, Lijuan L, Shouzhuo Y (2012) A sensitive and selective sensor-coated molecularly imprinted sol-gel film incorporating beta-cyclodextrin-multi-walled carbon nanotubes and cobalt nanoparticles-chitosan for oxacillin determination. Surf Interface Anal 44(3):334–341. doi:10.1002/sia.3807
Hu Y, Zhang Z, Zhang H, Luo L, Yao S (2012) Selective and sensitive molecularly imprinted sol-gel film-based electrochemical sensor combining mercaptoacetic acid-modified PbS nanoparticles with Fe3O4@Au-multi-walled carbon nanotubes-chitosan. J Solid State Electrochem 16(3):857–867. doi:10.1007/s10008-011-1434-4
Kan X, Liu T, Zhou H, Li C, Fang B (2010) Molecular imprinting polymer electrosensor based on gold nanoparticles for theophylline recognition and determination. Microchim Acta 171(3–4):423–429. doi:10.1007/s00604-010-0455-5
Li H, Xie C, Li S, Xu K (2012) Electropolymerized molecular imprinting on gold nanoparticle-carbon nanotube modified electrode for electrochemical detection of triazophos. Colloids Surf, B 89:175–181. doi:10.1016/j.colsurfb.2011.09.010
Lian W, Huang J, Yu J, Zhang X, Lin Q, He X, Xing X, Liu S (2012) A molecularly imprinted sensor based on beta-cyclodextrin incorporated multiwalled carbon nanotube and gold nanoparticles-polyamide amine dendrimer nanocomposites combining with water-soluble chitosan derivative for the detection of chlortetracycline. Food Control 26(2):620–627. doi:10.1016/j.foodcont.2012.02.023
Moreira FTC, Dutra RAF, Noronha JPC, Cunha AL, Sales MGF (2011) Artificial antibodies for troponin T by its imprinting on the surface of multiwalled carbon nanotubes: Its use as sensory surfaces. Biosens Bioelectron 28(1):243–250. doi:10.1016/j.bios.2011.07.026
Prasad BB, Kumar D, Madhuri R, Tiwari MP (2011) Sol-gel derived multiwalled carbon nanotubes ceramic electrode modified with molecularly imprinted polymer for ultra trace sensing of dopamine in real samples. Electrochim Acta 56(20):7202–7211. doi:10.1016/j.electacta.2011.04.090
Lee E, Park DW, Lee J-O, Kim DS, Lee BH, Kim BS (2008) Molecularly imprinted polymers immobilized on carbon nanotube. Colloids Surf, A 313:202–206. doi:10.1016/j.colsurfa.2007.04.093
Lee HY, Kim BS (2009) Grafting of molecularly imprinted polymers on iniferter-modified carbon nanotube. Biosens Bioelectron 25(3):587–591. doi:10.1016/j.bios.2009.03.040
Zhang XL, Zhang Y, Yin XF, Du BB, Zheng C, Yang HH (2013) A facile approach for preparation of molecularly imprinted polymers layer on the surface of carbon nanotubes. Talanta 105:403–408. doi:10.1016/j.talanta.2012.10.062
Hu Y, Li J, Zhang Z, Zhang H, Luo L, Yao S (2011) Imprinted sol–gel electrochemical sensor for the determination of benzylpenicillin based on Fe3O4@SiO2/multi-walled carbon nanotubes-chitosans nanocomposite film modified carbon electrode. Anal Chim Acta 698(1–2):61–68. doi:10.1016/j.aca.2011.04.054
Fu XC, Wu J, Nie L, Xie CG, Liu JH, Huang XJ (2012) Electropolymerized surface ion imprinting films on a gold nanoparticles/single-wall carbon nanotube nanohybrids modified glassy carbon electrode for electrochemical detection of trace mercury(II) in water. Anal Chim Acta 720:29–37. doi:10.1016/j.aca.2011.12.071
Zhao H, Chen Y, Tian J, Yu H, Quan X (2012) Selectively electrochemical determination of chloramphenicol in aqueous solution using molecularly imprinted polymer-carbon nanotubes-gold nanoparticles modified electrode. J Electrochem Soc 159(6):J231–J236. doi:10.1149/2.116206jes
Lu F, Li H, Sun M, Fan L, Qiu H, Li X, Luo C (2012) Flow injection chemiluminescence sensor based on core-shell magnetic molecularly imprinted nanoparticles for determination of sulfadiazine. Anal Chim Acta 718:84–91. doi:10.1016/j.aca.2011.12.054
Gao R, Su X, He X, Chen L, Zhang Y (2011) Preparation and characterisation of core-shell CNTs@MIPs nanocomposites and selective removal of estrone from water samples. Talanta 83(3):757–764. doi:10.1016/j.talanta.2010.10.034
Kong X, Gao R, He X, Chen L, Zhang Y (2012) Synthesis and characterization of the core-shell magnetic molecularly imprinted polymers Fe3O4@MIPs adsorbents for effective extraction and determination of sulfonamides in the poultry feed. J Chromatogr A 1245:8–16. doi:10.1016/j.chroma.2012.04.061
Prasad BB, Prasad A, Tiwari MP (2013) Multiwalled carbon nanotubes-ceramic electrode modified with substrate-selective imprinted polymer for ultra-trace detection of bovine serum albumin. Biosens Bioelectron 39(1):236–243. doi:10.1016/j.bios.2012.07.080
Yang M, Zhang Y, Lin S, Yang X, Fan Z, Yang L, Dong X (2013) Preparation of a bifunctional pyrazosulfuron-ethyl imprinted polymer with hydrophilic external layers by reversible addition-fragmentation chain transfer polymerization and its application in the sulfonylurea residue analysis. Talanta 114:143–151. doi:10.1016/j.talanta.2013.03.078
Hu Y, Li Y, Liu R, Tan W, Li G (2011) Magnetic molecularly imprinted polymer beads prepared by microwave heating for selective enrichment of beta-agonists in pork and pig liver samples. Talanta 84(2):462–470. doi:10.1016/j.talanta.2011.01.045
Sun X, He J, Cai G, Lin A, Zheng W, Liu X, Chen L, He X, Zhang Y (2010) Room temperature ionic liquid-mediated molecularly imprinted polymer monolith for the selective recognition of quinolones in pork samples. J Sep Sci 33(23–24):3786–3793. doi:10.1002/jssc.201000337
Walcarius A, Collinson MM (2009) Analytical chemistry with silica sol-gels: traditional routes to new materials for chemical analysis. Annu Rev Anal Chem 2:121–143. doi:10.1146/annurev-anchem-060908-155139
Suriyanarayanan S, Cywinski PJ, Moro AJ, Mohr GJ, Kutner W (2012) Chemosensors based on molecularly imprinted polymers. Top Curr Chem 325:165–265. doi:10.1007/128_2010_92
Malitesta C, Mazzotta E, Picca RA, Poma A, Chianella I, Piletsky SA (2012) MIP sensors–the electrochemical approach. Anal Bioanal Chem 402(5):1827–1846. doi:10.1007/s00216-011-5405-5
Lian W, Liu S, Yu J, Li J, Cui M, Xu W, Huang J (2013) Electrochemical sensor using neomycin-imprinted film as recognition element based on chitosan-silver nanoparticles/graphene-multiwalled carbon nanotubes composites modified electrode. Biosens Bioelectron 44:70–76. doi:10.1016/j.bios.2013.01.002
Shekarchizadeh H, Ensafi AA, Kadivar M (2013) Selective determination of sucrose based on electropolymerized molecularly imprinted polymer modified multiwall carbon nanotubes/glassy carbon electrode. Mater Sci Eng C 33(6):3553–3561. doi:10.1016/j.msec.2013.04.052
Minko S (2008) Grafting on solid surfaces: “Grafting to” and “Grafting from” methods. In: Stamm M (ed) Polymer surfaces and interfaces. Springer, Berlin, pp 215–234. doi:10.1007/978-3-540-73865-7_11
Lépinay S, Kham K, Millot M-C, Carbonnier B (2012) In-situ polymerized molecularly imprinted polymeric thin films used as sensing layers in surface plasmon resonance sensors: mini-review focused on 2010–2011. Chem Pap 66(5):340–351. doi:10.2478/s11696-012-0134-6
Prasad BB, Pandey I, Srivastava A, Kumar D, Tiwari MP (2013) Multiwalled carbon nanotubes-based pencil graphite electrode modified with an electrosynthesized molecularly imprinted nanofilm for electrochemical sensing of methionine enantiomers. Sens Actuators, B 176:863–874. doi:10.1016/j.snb.2012.09.050
Zhang Z, Zhang H, Hu Y, Yao S (2010) Synthesis and application of multi-walled carbon nanotubes-molecularly imprinted sol-gel composite material for on-line solid-phase extraction and high-performance liquid chromatography determination of trace Sudan IV. Anal Chim Acta 661(2):173–180. doi:10.1016/j.aca.2009.12.024
Zeng H, Wang Y, Liu X, Kong J, Nie C (2012) Preparation of molecular imprinted polymers using bi-functional monomer and bi-crosslinker for solid-phase extraction of rutin. Talanta 93:172–181. doi:10.1016/j.talanta.2012.02.008
Cai X, Li J, Zhang Z, Yang F, Dong R, Chen L (2014) Novel Pb2+ ion imprinted polymers based on ionic interaction via synergy of dual functional monomers for selective solid-phase extraction of Pb2+ in water samples. ACS Appl Mater Interfaces 6(1):305–313. doi:10.1021/am4042405
Wei Y, Qiu LH, Yu JCC, Lai EPC (2007) Molecularly imprinted solid phase extraction in a syringe needle packed with polypyrrole-encapsulated carbon nanotubes for determination of ochratoxin a in red wine. Food Sci Technol Int 13(5):375–380. doi:10.1177/1082013207085914
Zhang Z, Zhang H, Hu Y, Yang X, Yao S (2010) Novel surface molecularly imprinted material modified multi-walled carbon nanotubes as solid-phase extraction sorbent for selective extraction gallium ion from fly ash. Talanta 82(1):304–311. doi:10.1016/j.talanta.2010.04.038
Xiao D, Dramou P, Xiong N, He H, Yuan D, Dai H, Li H, He X, Peng J, Li N (2013) Preparation of molecularly imprinted polymers on the surface of magnetic carbon nanotubes with a pseudo template for rapid simultaneous extraction of four fluoroquinolones in egg samples. Analyst 138(11):3287–3296. doi:10.1039/c3an36755j
Ebrahimzadeh H, Moazzen E, Amini MM, Sadeghi O (2013) Novel ion imprinted polymer coated multiwalled carbon nanotubes as a high selective sorbent for determination of gold ions in environmental samples. Chem Eng J 215:315–321. doi:10.1016/j.cej.2012.11.031
Madrakian T, Ahmadi M, Afkhami A, Soleimani M (2013) Selective solid-phase extraction of naproxen drug from human urine samples using molecularly imprinted polymer-coated magnetic multi-walled carbon nanotubes prior to its spectrofluorometric determination. Analyst 138(16):4542–4549. doi:10.1039/c3an00686g
Zhang Z, Hu Y, Zhang H, Luo L, Yao S (2010) Layer-by-layer assembly sensitive electrochemical sensor for selectively probing L-histidine based on molecular imprinting sol-gel at functionalized indium tin oxide electrode. Biosens Bioelectron 26(2):696–702. doi:10.1016/j.bios.2010.06.062
Wu B, Wang Z, Xue Z, Zhou X, Du J, Liu X, Lu X (2012) A novel molecularly imprinted electrochemiluminescence sensor for isoniazid detection. Analyst 137(16):3644–3652. doi:10.1039/c2an35499c
Zhang M, Mullens C, Gorski W (2007) Coimmobilization of dehydrogenases and their cofactors in electrochemical biosensors. Anal Chem 79(6):2446–2450. doi:10.1021/ac061698n
Cai D, Ren L, Zhao H, Xu C, Zhang L, Yu Y, Wang H, Lan Y, Roberts MF, Chuang JH, Naughton MJ, Ren Z, Chiles TC (2010) A molecular-imprint nanosensor for ultrasensitive detection of proteins. Nat Nanotechnol 5(8):597–601. doi:10.1038/nnano.2010.114
Prasad BB, Srivastava A, Pandey I, Tiwari MP (2013) Electrochemically grown imprinted polybenzidine nanofilm on multiwalled carbon nanotubes anchored pencil graphite fibers for enantioselective micro-solid phase extraction coupled with ultratrace sensing of D- and L-methionine. J Chromatogr B Anal Technol Biomed Life Sci 912:65–74. doi:10.1016/j.jchromb.2012.10.010
Zhang D, Yu D, Zhao W, Yang Q, Kajiura H, Li Y, Zhou T, Shi G (2012) A molecularly imprinted polymer based on functionalized multiwalled carbon nanotubes for the electrochemical detection of parathion-methyl. Analyst 137(11):2629–2636. doi:10.1039/c2an35338e
Yang S, Yang R, Li G, Qu L, Li J, Yu L (2010) Nafion/multi-wall carbon nanotubes composite film coated glassy carbon electrode for sensitive determination of caffeine. J Electroanal Chem 639(1–2):77–82. doi:10.1016/j.jelechem.2009.11.025
Alizadeh T, Ganjali MR, Zare M, Norouzi P (2010) Development of a voltammetric sensor based on a molecularly imprinted polymer (MIP) for caffeine measurement. Electrochim Acta 55(5):1568–1574. doi:10.1016/j.electacta.2009.09.086
Sun JY, Huang KJ, Wei SY, Wu ZW, Ren FP (2011) A graphene-based electrochemical sensor for sensitive determination of caffeine. Colloids Surf B: Biointerfaces 84(2):421–426. doi:10.1016/j.colsurfb.2011.01.036
Santos WJR, Santhiago M, Yoshida IVP, Kubota LT (2012) Electrochemical sensor based on imprinted sol–gel and nanomaterial for determination of caffeine. Sens Actuators, B 166–167:739–745. doi:10.1016/j.snb.2012.03.051
Acknowledgments
This work was supported by the National Natural Science Foundation of China(Grant NO. 81402899), the Open Project of Key Laboratory of Modern Toxicology of the Ministry of Education (Grant NO. NMUMT201404), and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hao Dai and Deli Xiao equally contributed to this work and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Dai, H., Xiao, D., He, H. et al. Synthesis and analytical applications of molecularly imprinted polymers on the surface of carbon nanotubes: a review. Microchim Acta 182, 893–908 (2015). https://doi.org/10.1007/s00604-014-1376-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1376-5