Abstract
Tumor exosomes that inherit specific molecules from their parent cells are emerging as ideal biomarkers in cancer diagnostics. Most currently available exosome isolation and detection methods are time-consuming and non-specific; thus, rapid and specific exosome detection methods are needed both clinically and in research. Here, a dual-functional platform is reported composed of reversible conjunction and “off-on” signal responses. Fe3O4@SiO2@TiO2 particles with high affinity were applied to capture exosomes, and model exosomes could be isolated from solution within 20 min with a capture efficiency of 91.5%. An “on-off” fluorescence response PSMA aptasensor was constructed with improved selectivity to detect tumor exosomes by recording the fluorescence intensity with λex/em = 557/580 nm. The standard curve for detecting tumor exosomes with the aptasensor was calculated as y = 371.7x + 66.17, ranging from 0.05 to 1 × 104 particles/μL, with R2 = 0.9737, and a detection limit of 5 × 102 particles/μL in solution. This method was successfully applied to clinical samples, and the results showed better performance in distinguishing prostate cancer patients and healthy samples than the traditional nanoparticle-tracking analysis (NTA) method. This rapid and accurate detection method for prostate cancer may aid in rapid clinical diagnosis.
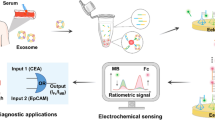
Graphical abstract

Integrating quickly TiO2-based isolation with sensitive and specific “on-off” detection of PCa exosomes.






Similar content being viewed by others
References
Filella X, Foj L (2016) Prostate cancer detection and prognosis: from prostate specific antigen (PSA) to exosomal biomarkers. Int J Mol Sci 17(11):1784. https://doi.org/10.3390/ijms17111784
Li Z, Yin J, Gao C, Qiu G, Meng A, Li Q (2019) The construction of electrochemical aptasensor based on coral-like poly-aniline and Au nano-particles for the sensitive detection of prostate specific antigen. Sensors Actuators B Chem 295:93–100. https://doi.org/10.1016/j.snb.2019.05.070
Duijvesz D, Versluis CY, van der Fels CA, Vredenbregt-van den Berg MS, Leivo J, Peltola MT, Bangma CH, Pettersson KS, Jenster G (2015) Immuno-based detection of extracellular vesicles in urine as diagnostic marker for prostate cancer. Int J Cancer 137(12):2869–2878. https://doi.org/10.1002/ijc.29664
Sun SY, Wang RM, Huang YD, Xu JL, Yao K, Liu WS, Cao YM, Qian K (2019) Design of hierarchical beads for efficient label-free cell capture. Small 15(34):902441. https://doi.org/10.1002/smll.201902441
Pei CC, Liu C, Wang Y, Cheng D, Li RX, Shu WK, Zhang CQ, Hu WL, Jin AH, Yang YN, Wan JJ (2020) FeOOH@metal-organic framework core-satellite nanocomposites for the serum metabolic fingerprinting of gynecological cancers. Angew Chem Int Edit 59(27):10831–10835. https://doi.org/10.1002/anie.202001135
Ibn Sina AA, Vaidyanathan R, Dey S, Carrascosa LG, Shiddiky MJA, Trau M (2016) Real time and label free profiling of clinically relevant exosomes. Sci Rep-Uk 6:30460. https://doi.org/10.1038/srep30460
Chen WQ, Li J, Wei XT, Fan YP, Qian HS, Li SQ, Xiang Y, Ding SJ (2020) Surface plasmon resonance biosensor using hydrogel-AuNP supramolecular spheres for determination of prostate cancer-derived exosomes. Microchim Acta 187(11):590. https://doi.org/10.1007/s00604-020-04573-4
Taylor DD, Gercel-Taylor C (2008) MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 110(1):13–21. https://doi.org/10.1016/j.ygyno.2008.04.033
Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, Widmark A (2009) Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Brit J Cancer 100(10):1603–1607. https://doi.org/10.1038/sj.bjc.6605058
Wang S, Zhang LQ, Wan S, Cansiz S, Cui C, Liu Y, Cai R, Hong CY, Teng IT, Shi ML, Wu Y, Dong YY, Tan WH (2017) Aptasensor with expanded nucleotide using DNA nanotetrahedra for electrochemical detection of cancerous exosomes. ACS Nano 11(4):3943–3949. https://doi.org/10.1021/acsnano.7b00373
Cimadamore A, Cheng M, Santoni M, Lopez-Beltran A, Battelli N, Massari F, Galosi AB, Scarpelli M, Montironi R (2018) New prostate cancer targets for diagnosis, imaging, and therapy: focus on prostate-specific membrane antigen. Front Oncol 8:653. https://doi.org/10.3389/fonc.2018.00653
Jin D, Yang F, Zhang YL, Liu L, Zhou YJ, Wang FB, Zhang GJ (2018) ExoAPP: exosome-oriented, aptamer nanoprobe-enabled surface proteins profiling and detection. Anal Chem 90(24):14402–14411. https://doi.org/10.1021/acs.analchem.8b03959
Mohan K, Donavan KC, Arter JA, Penner RM, Weiss GA (2013) Sub-nanomolar detection of prostate-specific membrane antigen in synthetic urine by synergistic, dual-ligand phage. J Am Chem Soc 135(20):7761–7767. https://doi.org/10.1021/ja4028082
Woo HK, Sunkara V, Park J, Kim TH, Han JR, Kim CJ, Choi HI, Kim YK, Cho YK (2017) Exodisc for rapid, size-selective, and efficient isolation and analysis of nanoscale extracellular vesicles from biological samples. ACS Nano 11(2):1360–1370. https://doi.org/10.1021/acsnano.6b06131
Boriachek K, Masud MK, Palma C, Phan HP, Yamauchi Y, Hossain MSA, Nguyen NT, Salomon C, Shiddiky MJA (2019) Avoiding pre-isolation step in exosome analysis: direct isolation and sensitive detection of exosomes using gold-loaded nanoporous ferric oxide nanozymes. Anal Chem 91(6):3827–3834. https://doi.org/10.1021/acs.analchem.8b03619
Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ (2012) Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 56(2):293–304. https://doi.org/10.1016/j.ymeth.2012.01.002
Boriachek K, Islam MN, Moller A, Salomon C, Nguyen NT, Hossain MSA, Yamauchi Y, Shiddiky MJA (2018) Biological functions and current advances in isolation and detection strategies for exosome nanovesicles. Small 14(6). https://doi.org/10.1002/smll.201702153
Thingholm TE, Larsen MR (2016) The use of titanium dioxide for selective enrichment of phosphorylated peptides. Methods Mol Biol 1355:135–146. https://doi.org/10.1007/978-1-4939-3049-4_9
Huang YR, Zhou QX, Xiao JP, Xie GH (2010) Determination of trace organophosphorus pesticides in water samples with TiO2 nanotubes cartridge prior to GC-flame photometric detection. J Sep Sci 33(14):2184–2190. https://doi.org/10.1002/jssc.201000147
Li Q, Wang Y, Yu G, Liu Y, Tang K, Ding C, Chen H, Yu S (2019) Fluorescent polymer dots and graphene oxide based nanocomplexes for “off-on” detection of metalloproteinase-9. Nanoscale 11(43):20903–20909. https://doi.org/10.1039/c9nr06557a
Li W, Yang JP, Wu ZX, Wang JX, Li B, Feng SS, Deng YH, Zhang F, Zhao DY (2012) A versatile kinetics-controlled coating method to construct uniform porous TiO2 shells for multifunctional core-shell structures. J Am Chem Soc 134(29):11864–11867. https://doi.org/10.1021/ja3037146
Ziaei P, Berkman CE, Norton MG (2018) Review: Isolation and detection of tumor-derived extracellular vesicles. ACS Appl Nano Mater 1(5):2004–2020. https://doi.org/10.1021/acsanm.8b00267
Zhang P, Zhou X, Zeng Y (2019) Multiplexed immunophenotyping of circulating exosomes on nano-engineered ExoProfile chip towards early diagnosis of cancer. Chem Sci 10(21):5495–5504. https://doi.org/10.1039/c9sc00961b
Gao FY, Jiao FL, Xia CS, Zhao Y, Ying WT, Xie YP, Guan XY, Tao M, Zhang YJ, Qin WJ, Qian XH (2019) A novel strategy for facile serum exosome isolation based on specific interactions between phospholipid bilayers and TiO2. Chem Sci 10(6):1579–1588. https://doi.org/10.1039/c8sc04197k
Pastor F, Kolonias D, Giangrande PH, Gilboa E (2010) Induction of tumour immunity by targeted inhibition of nonsense-mediated mRNA decay. Nature 465(7295):227–230. https://doi.org/10.1038/nature08999
Zhang JL, Shi JJ, Liu W, Zhang KX, Zhao HJ, Zhang HL, Zhang ZZ (2018) A simple, specific and “on-off” type MUC1 fluorescence aptasensor based on exosomes for detection of breast cancer. Sensors Actuators B Chem 276:552–559. https://doi.org/10.1016/j.snb.2018.08.056
Yu XC, He L, Pentok M, Yang HW, Yang YL, Li ZY, He NY, Deng Y, Li S, Liu TH, Chen XY, Luo HW (2019) An aptamer-based new method for competitive fluorescence detection of exosomes. Nanoscale 11(33):15589–15595. https://doi.org/10.1039/c9nr04050a
Funding
This work was supported by the National Key Research and Development Plan of China (2018YFF0212501) and National Natural Science Foundation of China (31470786).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 4988 kb)
Rights and permissions
About this article
Cite this article
Li, Q., Wang, Y., Ling, L. et al. Rapid and specific detection nanoplatform of serum exosomes for prostate cancer diagnosis. Microchim Acta 188, 283 (2021). https://doi.org/10.1007/s00604-021-04934-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04934-7