Abstract
Objective
The aim of the study was to investigate the putative neuroprotective effect of Nigella sativa oil (NSO) treatment against subarachnoid hemorrhage (SAH) in rats.
Methods
To induce SAH, rats were injected with 0.3 ml blood into their cisterna magna. Male Wistar albino rats were divided as control, vehicle-treated SAH, and NSO-treated (0.2 ml/kg, intraperitoneally) SAH groups. Forty-eight hours after SAH induction, neurological examination scores were recorded and the rats were decapitated. Brain tissue samples were taken for blood brain barrier permeability, brain water content, or determination of malondialdehyde (MDA) and glutathione (GSH) levels, myeloperoxidase (MPO), and Na+–K+–ATPase activities.
Results and discussion
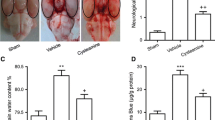
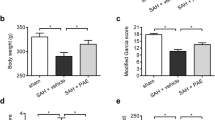
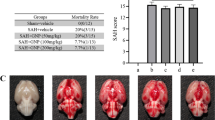
On the second day of SAH induction, neurological examination scores were increased in SAH groups, while SAH caused significant decreases in brain GSH content and Na+–K+–ATPase activity, which were accompanied with significant increases in MDA levels and MPO activity. The histological observation showed vasospasm of the basillary artery. On the other hand, NSO treatment markedly improved the neurological scores while all oxidant responses were prevented, implicating that NSO treatment may be of therapeutic use in preventing oxidative stress due to SAH.






Similar content being viewed by others
References
Ali BH, Blunden G (2003) Pharmacological and toxicological properties of Nigella sativa. Phytother Res 17:299–305
Ansar S, Larsen C, Maddahi A, Edvinsson L (2010) Subarachnoid hemorrhage induces enhanced expression of thromboxane A2 receptors in rat cerebral arteries. Brain Res 1316:163–172
Asano T (1999) Oxyhemoglobin as the principal cause of cerebral vasospasm: a holistic view of its actions. Crit Rev Neurosurg 9:303–318
Ayer R, Zhang J (2010) Connecting the early brain injury of aneurysmal subarachnoid hemorrhage to clinical practice. Turk Neurosurg 20:159–166
Ayer RE, Sugawara T, Chen W, Tong W, Zhang JH (2008) Melatonin decreases mortality following severe subarachnoid hemorrhage. J Pineal Res 44:197–204
Ayer RE, Zhang JH (2008) Oxidative stress in subarachnoid haemorrhage: significance in acute brain injury and vasospasm. Acta Neurochir Suppl 104:33–41
Badary OA, Taha RA, Gamal el-Din AM, Abdel-Wahab MH (2003) Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol 26:87–98
Bayrak O, Bavbek N, Karatas OF, Bayrak R, Catal F, Cimentepe E, Akbas A, Yildirim E, Unal D, Akcay A (2008) Nigella sativa protects against ischaemia/reperfusion injury in rat kidneys. Nephrol Dial Transplant 23:2206–2212
Beuge JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 53:302–311
Beutler E (1975) Glutathione in red blood cell metabolism. A manual of biochemical methods. Grune&Stratton, New York
Cahill J, Calvert JW, Solaroglu I, Zhang JH (2006) Vasospasm and p53-induced apoptosis in an experimental model of subarachnoid hemorrhage. Stroke 37:1868–1874
del Zoppo GJ, Hallenbeck JM (2000) Advances in the vascular pathophysiology of ischemic stroke. Thromb Res 98:73–81
del Zoppo GJ, von Kummer R, Hamann GF (1998) Ischaemic damage of brain microvessels: inherent risks for thrombolytic treatment in stroke. J Neurol Neurosurg Psychiatry 65:1–9
Delgado TJ, Brismar J, Svendgaard NA (1985) Subarachnoid haemorrhage in the rat: angiography and fluorescence microscopy of the major cerebral arteries. Stroke 16:595–602
Dietrich HH, Dacey RG Jr (2000) Molecular keys to the problems of cerebral vasospasm. Neurosurgery 46:517–530
Doczi T, Joo F, Adam G, Bozoky B, Szerdahelyi P (1986) Blood–brain barrier damage during the acute stage of subarachnoid hemorrhage, as exemplified by a new animal model. Neurosurgery 18:733–739
Doczi T, Joo F, Szerdahelyi P, Bodosi M (1987) Regulation of brain water and electrolyte contents: the possible involvement of central atrial natriuretic factor. Neurosurgery 21:454–458
El-Abhar HS, Abdallah DM, Saleh S (2003) Gastroprotective activity of Nigella sativa oil and its constituent, thymoquinone, against gastric mucosal injury induced by ischaemia/reperfusion in rats. J Ethnopharmacol 84:251–258
El-Dakhakhny M, Madi NJ, Lembert N, Ammon HP (2002) Nigella sativa oil, nigellone and derived thymoquinone inhibit synthesis of 5-lipoxygenase products in polymorphonuclear leukocytes from rats. J Ethnopharmacol 81:161–164
Erecinska M, Cherian S, Silver IA (2004) Energy metabolism in mammalian brain during development. Prog Neurobiol 73:397–445
Ersahin M, Sehirli O, Toklu HZ, Suleymanoglu S, Emekli-Alturfan E, Yarat A, Tatlidede E, Yegen BC, Sener G (2009) Melatonin improves cardiovascular function and ameliorates renal, cardiac and cerebral damage in rats with renovascular hypertension. J Pineal Res 47:97–106
Ersahin M, Toklu HZ, Cetinel S, Yuksel M, Erzik C, Berkman MZ, Yegen BC, Sener G (2010) Alpha lipoic acid alleviates oxidative stress and preserves blood brain permeability in rats with subarachnoid hemorrhage. Neurochem Res 35:418–428
Ersahin M, Toklu HZ, Erzik C, Cetinel S, Akakin D, Velioglu-Ogunc A, Tetik S, Ozdemir ZN, Sener G, Yegen BC (2010) The anti-inflammatory and neuroprotective effects of ghrelin in subarachnoid hemorrhage-induced oxidative brain damage in rats. J Neurotrauma 27:1143–1155
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Gaetani P, Lombardi D (1992) Brain damage following subarachnoid hemorrhage: the imbalance between anti-oxidant systems and lipid peroxidative processes. J Neurosurg Sci 36:1–10
Gaetani P, Pasqualin A, Rodriguez y Baena R, Borasio E, Marzatico F (1998) Oxidative stress in the human brain after subarachnoid hemorrhage. J Neurosurg 89:748–754
Garcia JH, Liu KF, Yoshida Y, Lian J, Chen S, del Zoppo GJ (1994) Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat). Am J Pathol 144:188–199
Germano A, d’Avella D, Cicciarello R, Hayes RL, Tomasello F (1992) Blood–brain barrier permeability changes after experimental subarachnoid hemorrhage. Neurosurgery 30:882–886
Haklar GYM, Yalçın AS (1998) Chemiluminescence in the measurement of free radicals. Theory and application on a tissue injury model. Marmara Med J 11:56–60
Hillegass LM, Griswold DE, Brickson B, Albrightson-Winslow C (1990) Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods 24:285–295
Hosseinzadeh H, Parvardeh S (2004) Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine 11:56–64
Hosseinzadeh H, Parvardeh S, Asl MN, Sadeghnia HR, Ziaee T (2007) Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia–reperfusion injury in rat hippocampus. Phytomedicine 14:621–627
Hosseinzadeh H, Parvardeh S, Nassiri-Asl M, Mansouri MT (2005) Intracerebroventricular administration of thymoquinone, the major constituent of Nigella sativa seeds, suppresses epileptic seizures in rats. Med Sci Monit 11:BR106–BR110
Ismail M, Al-Naqeep G, Chan KW (2010) Nigella sativa thymoquinone-rich fraction greatly improves plasma antioxidant capacity and expression of antioxidant genes in hypercholesterolemic rats. Free Radic Biol Med 48:664–672
Kanter M (2008) Effects of Nigella sativa and its major constituent, thymoquinone on sciatic nerves in experimental diabetic neuropathy. Neurochem Res 33:87–96
Kanter M (2008) Nigella sativa and derived thymoquinone prevents hippocampal neurodegeneration after chronic toluene exposure in rats. Neurochem Res 33:579–588
Kanter M, Coskun O, Kalayci M, Buyukbas S, Cagavi F (2006) Neuroprotective effects of Nigella sativa on experimental spinal cord injury in rats. Hum Exp Toxicol 25:127–133
Karaoglan A, Akdemir O, Barut S, Kokturk S, Uzun H, Tasyurekli M, Colak A (2008) The effects of resveratrol on vasospasm after experimental subarachnoidal hemorrhage in rats. Surg Neurol 70:337–343
Kim YK, Lee SH, Goldinger JM, Hong SK (1986) Effect of ethanol on organic ion transport in rabbit kidney. Toxicol Appl Pharmacol 86:411–420
Kiris T, Karasu A, Yavuz C, Erdem T, Unal F, Hepgul K, Baloglu H (1999) Reversal of cerebral vasospasm by the nitric oxide donor SNAP in an experimental model of subarachnoid haemorrhage. Acta Neurochir (Wien) 141:1323–1328, discussion 1328–1329
Kozniewska E, Michalik R, Rafalowska J, Gadamski R, Walski M, Frontczak-Baniewicz M, Piotrowski P, Czernicki Z (2006) Mechanisms of vascular dysfunction after subarachnoid hemorrhage. J Physiol Pharmacol 57(Suppl 11):145–160
Kurella E, Kukley M, Tyulina O, Dobrota D, Matejovicova M, Mezesova V, Boldyrev A (1997) Kinetic parameters of Na/K–ATPase modified by free radicals in vitro and in vivo. Ann NY Acad Sci 834:661–665
Lowry OHRN, Farr AL, Randall RJ (1951) Protein measurements with the folin phenol reagent. J Biol Chem 193:265–275
Marzatico F, Gaetani P, Cafe C, Spanu G, Rodriguez y Baena R (1993) Antioxidant enzymatic activities after experimental subarachnoid hemorrhage in rats. Acta Neurol Scand 87:62–66
Ohara Y, Peterson TE, Harrison DG (1993) Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 91:2546–2551
Park IS, Meno JR, Witt CE, Chowdhary A, Nguyen TS, Winn HR, Ngai AC, Britz GW (2009) Impairment of intracerebral arteriole dilation responses after subarachnoid hemorrhage. Laboratory investigation. J Neurosurg 111:1008–1013
Petzold GC, Einhaupl KM, Dirnagl U, Dreier JP (2003) Ischemia triggered by spreading neuronal activation is induced by endothelin-1 and hemoglobin in the subarachnoid space. Ann Neurol 54:591–598
Reading HW, Isbir T (1980) The role of cation-activated ATPases in transmitter release from the rat iris. Q J Exp Physiol Cogn Med Sci 65:105–116
Reiter RJ, Tan DX, Manchester LC, Qi W (2001) Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys 34:237–256
Sabri M, Kawashima A, Ai J, Macdonald RL (2008) Neuronal and astrocytic apoptosis after subarachnoid hemorrhage: a possible cause for poor prognosis. Brain Res 1238:163–171
Sayed-Ahmed MM, Nagi MN (2007) Thymoquinone supplementation prevents the development of gentamicin-induced acute renal toxicity in rats. Clin Exp Pharmacol Physiol 34:399–405
Schubert GA, Thome C (2008) Cerebral blood flow changes in acute subarachnoid hemorrhage. Front Biosci 13:1594–1603
Sethi G, Ahn KS, Aggarwal BB (2008) Targeting nuclear factor-kappa B activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol Cancer Res 6:1059–1070
Stark G (2005) Functional consequences of oxidative membrane damage. J Membr Biol 205:1–16
Tang WH, Chen Z, Liu Z, Zhang JH, Xi G, Feng H (2008) The effect of ecdysterone on cerebral vasospasm following experimental subarachnoid hemorrhage in vitro and in vivo. Neurol Res 30:571–580
Terzi A, Coban S, Yildiz F, Ates M, Bitiren M, Taskin A, Aksoy N (2010) Protective effects of Nigella sativa on intestinal ischemia–reperfusion injury in rats. J Invest Surg 23:21–27
Toklu HZ, Hakan T, Biber N, Solakoglu S, Ogunc AV, Sener G (2009) The protective effect of alpha lipoic acid against traumatic brain injury in rats. Free Radic Res 43:658–667
Toklu HZ, Uysal MK, Kabasakal L, Sirvanci S, Ercan F, Kaya M (2009) The effects of riluzole on neurological, brain biochemical, and histological changes in early and late term of sepsis in rats. J Surg Res 152:238–248
Wyse AT, Streck EL, Barros SV, Brusque AM, Zugno AI, Wajner M (2000) Methylmalonate administration decreases Na+, K+–ATPase activity in cerebral cortex of rats. NeuroReport 11:2331–2334
Yang Y, Zhang XJ, Yin J, Li LT (2008) Brain damage related to hemorrhagic transformation following cerebral ischemia and the role of K ATP channels. Brain Res 1241:168–175
Yang YB, Piao YJ (2003) Effects of resveratrol on secondary damages after acute spinal cord injury in rats. Acta Pharmacol Sin 24:703–710
Yildiz F, Coban S, Terzi A, Ates M, Aksoy N, Cakir H, Ocak AR, Bitiren M (2008) Nigella sativa relieves the deleterious effects of ischemia reperfusion injury on liver. World J Gastroenterol 14:5204–5209
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erşahin, M., Toklu, H.Z., Akakin, D. et al. The effects of Nigella sativa against oxidative injury in a rat model of subarachnoid hemorrhage. Acta Neurochir 153, 333–341 (2011). https://doi.org/10.1007/s00701-010-0853-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-010-0853-9