Abstract
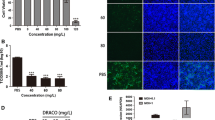
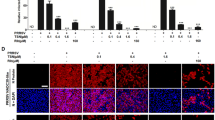
Porcine reproductive and respiratory syndrome virus (PRRSV) is a devastating viral pathogen of swine that causes huge financial losses in the pig industry worldwide. Ivermectin is known to be a potent inhibitor of importin α/β-mediated nuclear transport and exhibits antiviral activity towards several RNA viruses by blocking the nuclear trafficking of viral proteins. Although PRRSV replication occurs exclusively in the cytoplasm of infected cells, the nucleocapsid (N) protein has been shown to distinctly localize in the nucleus and nucleolus throughout infection. Here, we sought to assess whether ivermectin suppresses PRRSV replication in cultured porcine alveolar macrophage (PAM) cells and to investigate the effect of ivermectin on the subcellular localization of the PRRSV N protein. Our data demonstrate that ivermectin treatment inhibits PRRSV infection in PAM-pCD163 cells in a dose-dependent manner. The antiviral activity of ivermectin on PRRSV replication was most effective when cells were treated during the early stage of infection. Treatment of PRRSV-infected cells with ivermectin significantly suppressed viral RNA synthesis, viral protein expression, and progeny virus production. However, immunofluorescence and cell fractionation assays revealed that ivermectin was incapable of disrupting the nuclear localization of the N protein, both in PRRSV-infected PAM-pCD163 cells and in PAM cells stably expressing the PRRSV N protein. This finding suggests that an alternative mechanism of action accounts for the ability of ivermectin to diminish PRRSV replication. Taken together, our results suggest that ivermectin is an invaluable therapeutic or preventative agent against PRRSV infection.







Similar content being viewed by others
References
An TQ, Tian ZJ, Leng CL, Peng JM, Tong GZ (2011) Highly pathogenic porcine reproductive and respiratory syndrome virus, Asia. Emerg Infect Dis 17:1782–1784
Babalola OE (2011) Ocular onchocerciasis: current management and future prospects. Clin Ophthalmol 5:1479–1491
Cai Y, Liu Y, Zhang X (2007) Suppression of coronavirus replication by inhibition of the MEK signaling pathway. J Virol 81:446–456
Caly L, Wagstaff KM, Jans DA (2012) Nuclear trafficking of proteins from RNA viruses: potential target for antivirals? Antiviral Res 95:202–206
Chen Z, Lawson S, Sun Z, Zhou X, Guan X, Christopher-Hennings J, Nelson EA, Fang Y (2010) Identification of two auto-cleavage products of nonstructural protein 1 (nsp1) in porcine reproductive and respiratory syndrome virus infected cells: nsp1 function as interferon antagonist. Virology 398:87–97
Fang Y, Snijder EJ (2010) The PRRSV replicase: exploring the multifunctionality of an intriguing set of nonstructural proteins. Virus Res 154:61–76
Fang Y, Treffers EE, Li Y, Tas A, Sun Z, van der Meer Y, de Ru AH, van Veelen PA, Atkins JF, Snijder EJ, Firth AE (2012) Efficient -2 frameshifting by mammalian ribosomes to synthesize an additional arterivirus protein. Proc Natl Acad Sci USA 109:E2920–E2928
Firth AE, Zevenhoven-Dobbe JC, Wills NM, Go YY, Balasuriya UB, Atkins JF, Snijder EJ, Posthuma CC (2011) Discovery of a small arterivirus gene that overlaps the GP5 coding sequence and is important for virus production. J Gen Virol 92:1097–1106
Fulcher AJ, Jans DA (2011) Regulation of nucleocytoplasmic trafficking of viral proteins: an integral role in pathogenesis? Biochim Biophys Acta 1813:2176–2190
Ginisty H, Sicard H, Roger B, Bouvet P (1999) Structure and functions of nucleolin. J Cell Sci 112:761–772
Gómez-Laguna J, Salguero FJ, Pallarés FJ, Carrasco L (2013) Immunopathogenesis of porcine reproductive and respiratory syndrome in the respiratory tract of pigs. Vet J 195:148–155
Han M, Du Y, Song C, Yoo D (2013) Degradation of CREB-binding protein and modulation of type I interferon induction by the zinc finger motif of the porcine reproductive and respiratory syndrome virus nsp1α subunit. Virus Res 172:54–65
Holtkamp DJ, Kliebenstein JB, Neumann EJ, Zimmerman J, Rotto H, Yoder TK, Wang C, Yeske P, Mowrer C, Haley C (2013) Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J Swine Health Prod 21:72–84
Johnson CR, Griggs TF, Gnanandarajah J, Murtaugh MP (2011) Novel structural protein in porcine reproductive and respiratory syndrome virus encoded by an alternative ORF5 present in all arteriviruses. J Gen Virol 92:1107–1116
Kim O, Sun Y, Lai FW, Song C, Yoo D (2010) Modulation of type I interferon induction by porcine reproductive and respiratory syndrome virus and degradation of CREB-binding protein by non-structural protein 1 in MARC-145 and HeLa cells. Virology 402:315–326
Lee C, Calvert JG, Welch SK, Yoo D (2005) A DNA-launched reverse genetics system for porcine reproductive and respiratory syndrome virus reveals that homodimerization of the nucleocapsid protein is essential for virus infectivity. Virology 331:47–62
Lee C, Hodgins D, Calvert JG, Welch SK, Jolie R, Yoo D (2006) Mutations within the nuclear localization signal of the porcine reproductive and respiratory syndrome virus nucleocapsid protein attenuate virus replication. Virology 346:238–250
Lee YJ, Lee C (2010) Porcine reproductive and respiratory syndrome virus replication is suppressed by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. Virus Res 152:50–58
Lee YJ, Park CK, Nam E, Kim SH, Lee OS, Lee DS, Lee C (2010) Generation of a porcine alveolar macrophage cell line for the growth of porcine reproductive and respiratory syndrome virus. J Virol Methods 163:410–415
Li Y, Tas A, Snijder EJ, Fang Y (2012) Identification of porcine reproductive and respiratory syndrome virus ORF1a-encoded non-structural proteins in virus-infected cells. J Gen Virol 93:829–839
Li Y, Treffers EE, Napthine S, Tas A, Zhu L, Sun Z, Bell S, Mark BL, van Veelen PA, van Hemert MJ, Firth AE, Brierley I, Snijder EJ, Fang Y (2014) Transactivation of programmed ribosomal frameshifting by a viral protein. Proc Natl Acad Sci USA 111:E2172–E2181
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25:402–408
Lundberg L, Pinkham C, Baer A, Amaya M, Narayanan A, Wagstaff KM, Jans DA, Kehn-Hall K (2013) Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan equine encephalitis virus replication. Antiviral Res 100:662–672
Lunney JK, Benfield DA, Rowland RR (2010) Porcine reproductive and respiratory syndrome virus: an update on an emerging and re-emerging viral disease of swine. Virus Res 154:1–6
Mastrangelo E, Pezzullo M, De Burghgraeve T, Kaptein S, Pastorino B, Dallmeier K, de Lamballerie X, Neyts J, Hanson AM, Frick DN, Bolognesi M, Milani M (2012) Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J Antimicrob Chemother 67:1884–1894
Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, Green AL, Zimmerman JJ (2005) Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc 227:385–392
Rowland RR, Kervin R, Kuckleburg C, Sperlich A, Benfield DA (1999) The localization of porcine reproductive and respiratory syndrome virus nucleocapsid protein to the nucleolus of infected cells and identification of a potential nucleolar localization signal sequence. Virus Res 64:1–12
Rowland RR, Schneider P, Fang Y, Wootton S, Yoo D, Benfield DA (2003) Peptide domains involved in the localization of the porcine reproductive and respiratory syndrome virus nucleocapsid protein to the nucleolus. Virology 316:135–145
Sagong M, Lee C (2011) Porcine reproductive and respiratory syndrome virus nucleocapsid protein modulates interferon-β production by inhibiting IRF3 activation in immortalized porcine alveolar macrophages. Arch Virol 156:2187–2195
Snijder EJ, Spaan WJM (2007) Arteriviridae. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (eds) Fields Virology, 5th edn. Lippincott Williams & Wilkins, Philadelphia, pp 1337–1361
Snijder EJ, Kikkert M, Fang Y (2013) Arterivirus molecular biology and pathogenesis. J Gen Virol 94:2141–2163
Srivastava M, Pollard HB (1999) Molecular dissection of nucleolin’s role in growth and cell proliferation: new insights. FASEB J 13:1911–1922
Strycharz JP, Yoon KS, Clark JM (2008) A new ivermectin formulation topically kills permethrin-resistant human head lice (Anoplura: Pediculidae). J Med Entomol 45:75–81
Tay MY, Fraser JE, Chan WK, Moreland NJ, Rathore AP, Wang C, Vasudevan SG, Jans DA (2013) Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antiviral Res 99:301–306
Tong GZ, Zhou YJ, Hao XF, Tian ZJ, An TQ, Qiu HJ (2007) Highly pathogenic porcine reproductive and respiratory syndrome, China. Emerg Infect Dis 13:1434–1436
Victoria J, Trujillo R (2001) Topical ivermectin: a new successful treatment for scabies. Pediatr Dermatol 18:63–65
Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA (2012) Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J 443:851–856
Wootton SK, Yoo D (2003) Homo-oligomerization of the porcine reproductive and respiratory syndrome virus nucleocapsid protein and the role of disulfide linkages. J Virol 77:4546–4557
Zhang Y, Li H, Peng G, Zhang Y, Gao X, Xiao S, Cao S, Chen H, Song Y (2015) Mutational analysis of the functional sites in porcine reproductive and respiratory syndrome virus non-structural protein 10. J Gen Virol 96:547–552
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2013R1A2A2A01004355) and Technology Development Program for Bio-industry, Ministry for Agriculture, Food and Rural Affairs, Republic of Korea (311007-05-1-HD120).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
705_2015_2653_MOESM1_ESM.tif
Fig. A1. Nuclear and cytoplasmic fractionation of PRRSV-infected PAM cells treated with ivermectin. Each nuclear and cytosolic fraction was prepared from PAM-pCD163 cells infected with PRRSV and treated with different concentrations of ivermectin for 48 h (A) or with a constant concentration of ivermectin (10 μM) at the indicated time points post-infection (B). These fractions were subjected to western blot analysis with an antibody specific for PRRSV N protein (top panel), α-tubulin as a cytosol protein marker (middle panel), or SP1 as a nuclear protein marker (bottom panel) (TIFF 11586 kb)
Rights and permissions
About this article
Cite this article
Lee, Y.J., Lee, C. Ivermectin inhibits porcine reproductive and respiratory syndrome virus in cultured porcine alveolar macrophages. Arch Virol 161, 257–268 (2016). https://doi.org/10.1007/s00705-015-2653-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-015-2653-2