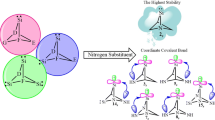
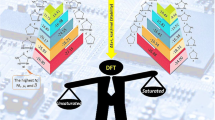
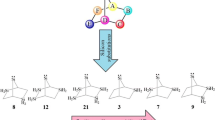
Abstract
Cyclonona-3,5,7-trienylidene appears as boat-shaped transition state for having a negative force constant, while its singlet state exhibits less stability than the corresponding triplet state. Succeeding the quest for the largest unsaturated stable carbene-like species, theoretical investigations coupled with suitable isodesmic reactions are used to examine the effects of α,αʹ-tetrahalo groups on the thermodynamic along with kinetic viabilities of nine-membered cyclic silylenes. All the singlet and triplet silylenes appear as boat-shaped minima for having positive force constants on their potential energy surfaces and singlet states emerge as ground state, exhibiting more stability than their corresponding triplet states. The order of stability estimated by singlet (S)–triplet (T) energy separation (ΔES–T = ET − ES) emerges as α,αʹ-tetrahydrocarbene < α,αʹ-tetrahydrosilylene < α,αʹ-tetrafluorosilylene < α,αʹ-tetraiodosilylene < α,αʹ-tetrachlorosilylene < α,αʹ-tetrabromosilylene. This research specifies band gap (ΔEHOMO–LUMO) of scrutinized silylenes with this order. Hence, singlet 2,2,9,9-tetrabromosilacyclonona-3,5,7-trienylidene exists as the most stable species. From both thermodynamic and kinetic points of view, this species is more stable than synthesized silylene by Kira. It shows the highest heat of dehydrogenation through isodesmic reaction. The NBO analysis provides significant evidences for the stability of it through positive hyperconjugation, negative hyperconjugation, as well as mesomeric effects.
Graphic abstract






Similar content being viewed by others
References
Kassaee MZ, Zandi H, Haerizade BN, Ghambarian M (2012) Comput Theor Chem 1001:39
Momeni MR, Shakib FA (2011) Organomet 30:5027
Ayoubi-Chianeh M, Kassaee MZ, Ashenagar S, Cummings PT (2019) J Phys Org Chem 32:e3956
Brück A, Gallego D, Wang W, Irran E, Driess M, Hartwig JF (2012) Angew Chem Int Ed 51:11478
Li J, Merkel S, Henn J, Meindl K, Döring A, Roesky HW, Ghadwal RS, Stalke D (2010) Inorg Chem 49:775
Yang W, Fu H, Wang H, Chen M, Ding Y, Roesky HW, Jana A (2009) Inorg Chem 48:5058
Yamada T, Mawatari A, Tanabe M, Osakada K, Tanase T (2009) Angew Chem 121:576
Blom B, Enthaler S, Inoue S, Irran E, Driess M (2013) J Am Chem Soc 135:6703
Tan G, Blom B, Gallego D, Driess M (2013) Organometallics 33:363
Blom B, Stoelzel M, Driess M (2013) Chem Eur J 19:40
Stoelzel M, Präsang C, Blom B, Driess M (2013) Aust J Chem 66:1163
Protchenko AV, Birjkumar KH, Dange D, Schwarz AD, Vidovic D, Jones C, Kaltsoyannis N, Mountford P, Aldridge S (2012) J Am Chem Soc 134:6500
Rekken BD, Brown TM, Fettinger JC, Tuononen HM, Power PP (2012) J Am Chem Soc 134:6504
Asay M, Inoue S, Driess M (2011) Angew Chem Int Ed 50:9589
Sasamori T, Tokitoh N (2005) In: King RB (ed) Encyclopedia of Inorganic Chemistry II. Wiley, Chichester, p 1698
Slipchenko LV, Krylov AI (2002) J Chem Phys 117:4694
Denk M, Lennon R, Hayashi R, West R, Haaland A, Belyakov H, Verne P, Wagner M, Metzler N (1994) J Am Chem Soc 116:2691
Gehrhus B, Lappert MF, Heinicke J, Boese R, Bläser D (1995) J Chem Soc Chem Commun 19:1931–1932
West R, Denk M (1996) Pure Appl Chem 68:785
Heinicke J, Oprea A, Kindermann MK, Karpati T, Nyulaszi L, Veszpremi T, Hitchcock PB, Lappert MF, Maciejewski H (1998) Organometallics 17:5599
Kira M, Ishida S, Iwamoto T, Kabuto C (1999) J Am Chem Soc 121:9722
Driess M, Yao S, Brym M, Wüllen C, Lentz D (2006) J Am Chem Soc 128:9628
Kassaee MZ, Koohi M (2013) J Phys Org Chem 26:540
Kassaee MZ, Koohi M, Mohammadi R, Ghavami M (2013) J Phys Org Chem 26:908
Koohi M, Kassaee MZ, Haerizade BN, Ghavami M, Ashenagar S (2015) J Phys Org Chem 28:514
Naderi F, Bagheri R, Yari M (2013) J Phys Theor Chem 9:281
Mekky ABH, Elhaes HG, El-Okr MM, Ibrahim MA (2015) J Nanomater Mol Nanotechnol 4:2
Mizuhata Y, Sasamori T, Tokitoh N (2009) Chem Rev 109:3479
Govindarajan M, Karabacak M, Suvitha A, Periandy S (2012) Spectrochim Acta A Mol Biomol Spectrosc 89:137
Ruiz-Espinoza A, Ramos E, Salcedo R (2013) Comput Theor Chem 1016:36
Dheivamalar S, Sugi L, Ambigai K (2016) Comput Chem 4:17
Dheivamalar S, Sugi L (2015) Spectrochim Acta A Mol Biomol Spectrosc 151:687
Hoffmann R, Schleyer PR, Schaefer HF (2008) Angew Chem Int Ed 47:7164
Nemirowski A (2007) Schreiner PR). J Org Chem 72:9533–9540
Kassaee MZ, Koohi M (2005) J Mol Struct (THEOCHEM) 755:91
Kassaee MZ, Koohi M, Arshadi S (2005) J Mol Struct (THEOCHEM) 724:61
Kassaee MZ, Koohi M (2007) J Mol Struct (THEOCHEM) 815:21
Kassaee MZ, Koohi M (2007) J Mol Struct (THEOCHEM) 810:53
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347
Sobolewski AL, Domcke W (2002) J Phys Chem A 106:4158
Becke AD (1988) Phys Rev A 38:3098
Becke AD (1993) J Chem Phys 98:5648
Becke AD (1996) J Chem Phys 104:1040
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Krishna R, Frisch MJ, Pople JA (1980) J Chem Phys 72:4244
Zhao Y, Truhlar DG (2008) Theor Chem Account 120:215
Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pople JA (1982) J Chem Phys 77:3654
Clark T, Chandrasekhar J, Spitznagel GW, Schleyer PR (1983) J Comput Chem 4:294
Frisch MJ, Pople JA, Binkley JS (1984) J Chem Phys 80:3265
Schlegel HB, Frisch MJ (1995) Int J Quantum Chem 54:83
Kendall RA, Dunning TH Jr, Harrison RJ (1992) J Chem Phys 96:6796
Hehre WJ, Radom L, PvR Schleyer, Pople JA (1986) Ab Initio Molecular Orbital Theory. John Wiley & Sons, New York
Foresman JB, Frisch A (1996) Exploring Chemistry with Electronic structure Methods. Gaussian Inc, Pittsburgh
Glendening ED, Reed AE, Carpenter JE, Weinhold F NBO Version 3.1
Weinhold F, Glendening ED, NBO Version 7.0
Weinhold F (2012) J Comput Chem 33:2363
Glendening ED, Landis CR, Weinhold F (2012) Wiley Interdiscip Rev Comput Mol Sci 2:1
Domingo LR, Pérez P (2011) Org Biomol Chem 9:7168
Domingo LR, Chamorro E, Pérez P (2008) J Org Chem 73:4615
Parr RG, Szentpaly L, Liu S (1999) J Am Chem Soc 121:1922
Pearson RG (1989) J Org Chem 54:1423
Chattaraj PK, Giri S (2007) J Phys Chem A 111:11116
Padmanabhan J, Parthasarathi R, Subramanian V, Chattaraj PK (2007) J Phys Chem A 111:1358
Acknowledgements
This research is financially supported by Technical and Vocational University of Tehran, Dr. Shariaty College, Tehran, and North Tehran Branch, Islamic Azad University, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
706_2019_2537_MOESM1_ESM.docx
Supplementary material 1 The calculated sum of electronic and thermal enthalpy (Htot), sum of electronic and thermal free energy (Gtot), changes of enthalpy (ΔHS-T), changes of free energy (ΔGS-T), polarizability (αxx, αyy, αzz, and <α>), nucleophilicity index (N), global electrophilicity (ω), chemical potential (μ), global hardness (η), electronegativity (χ), global softness (S), maximum electronic charge (ΔNmax), the second order perturbation theory analysis of Fock Matrix in NBO basis including stabilization energies E(2) corresponding to the most important charge transfer interactions (donor-acceptor), C-D-C angle (D being the divalent, carbene-like atom), bond lengths, XYZ Cartesian coordinates, MEP maps, and shapes of selected frontier molecular orbitals for scrutinized silylenes (27 pages) (DOCX 34341 kb)
Rights and permissions
About this article
Cite this article
Koohi, M., Bastami, H. Substituent effects on stability, MEP, NBO analysis, and reactivity of 2,2,9,9-tetrahalosilacyclonona-3,5,7-trienylidenes, at density functional theory. Monatsh Chem 151, 11–23 (2020). https://doi.org/10.1007/s00706-019-02537-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-019-02537-w