Abstract
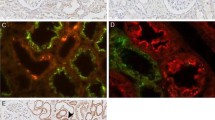
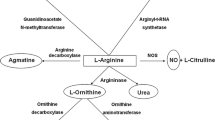
The kidney plays a key role in arginine metabolism. Arginine production is controlled by argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL) which metabolize citrulline and aspartate to arginine and fumarate whereas arginine consumption is dependent on arginine:glycine amidinotransferase (GAT), which mediates creatine and ornithine synthesis. Histological and biochemical techniques have been used to study the distribution and activity of these enzymes in anatomically dissected segments, in isolated fragments of tubules and in whole tissues. ASS and ASL mRNAs and proteins are expressed in the proximal tubule. Within this nephron segment, the proximal convoluted tubule has a higher arginine synthesis capacity than the proximal straight tubules. Furthermore, this arginine-synthesizing portion of the nephron matches perfectly with the site of citrulline reabsorption from the glomerular filtrate. The kidney itself can produce citrulline from methylated arginine, but this capacity is limited. Therefore, intestinal citrulline synthesis is required for renal arginine production. Although the proximal convoluted tubule also expresses a significant amount of GAT, only 10% of renal arginine synthesis is metabolized to guanidinoacetic acid, possibly because GAT has a mitochondrial localization. Kidney arginase (AII) is expressed in the cortical and outer medullary proximal straight tubules and does not degrade significant amounts of newly synthesized arginine. The data presented in this review identify the proximal convoluted tubule as the main site of endogenous arginine biosynthesis.








Similar content being viewed by others
References
Al Banchaabouchi M, Marescau B, Possemiers I, D’Hooge R, Levillain O, De Deyn PP (2000) N-G, N-G-dimethylarginine and N-G, N′G-dimethylarginine in renal insufficiency. Pflügers Arch Eur J Physiol 439(5):524–531
Anderson PA, Baker DH, Corbin JE (1979) Lysine and arginine requirements of the domestic cats. J Nutr 109:1368–1372
Aperia A, Broberger O, Larsson A, Snellman K (1979) Studies of renal urea cycle enzymes. I. Renal concentrating ability and urea cycle enzymes in the rat during protein deprivation. Scand J Clin Lab Invest 39:329–336
Archibald RM (1944) Determination of citrulline and allantoin and demonstration of citrulline in blood plasma. J Biol Chem 156(1):121–142
Bachmann S, Bosse HM, Mundel P (1995) Topography of nitric oxid synthesis by localizing constitutive NO synthases in mammalian kidney. Am J Physiol Renal Fluid Electrolyte Physiol 268(5 Pt 2):F884–F898
Baker DH, Czarnecki-Maulden GL (1991) Comparative nutrition of cats and dogs. Ann Rev Nutr 11:239–263
Balaban RS, Soltoff SP, Storey JM, Mandel LJ (1980) Improved renal cortical tubule suspension: spectrophotometric study of O2 delivery. Am J Physiol Renal Physiol 238(1):F50–59
Bankir L, Bouby N, Trinh-Trang-Tan MM (1987) Heterogeneity of nephron anatomy. Kidney Int 31(suppl 20):S-25–S-39
Beaven MA, Wilcox G, Terpstra GK (1978) A microprocedure for the measurement of 14CO2 release from [14C]carboxyl-labeled amino acids. Anal Biochem 84(2):638–641
Boelens PG, van Leeuwen PA, Dejong CH, Deutz NE (2005) Intestinal renal metabolism of l-citrulline and l-arginine following enteral or parenteral infusion of l-alanyl-l-[2, 15N]glutamine or l-[2, 15N]glutamine in mice. Am J Physiol Gastrointest Liver Physiol 289(4):G679–G685
Borsook H, Dubnoff JW (1941a) The conversion of citrulline to arginine in kidney. J Biol Chem 140(3):717–738
Borsook H, Dubnoff JW (1941b) The formation of glycocyamine in animal tissues. J Biol Chem 138:389–403
Bouby N, Hassler C, Parvy P, Bankir L (1993) Renal synthesis of arginine in chronic renal failure: in vivo and in vitro studies in rats with 5/6 nephrectomy. Kidney Int 44:676–683
Brosnan ME, Brosnan JT (2004) Renal arginine metabolism. J Nutr 134(10):2791S–2795S
Burg MB, Orloff J (1962) Oxygen consumption and active transport in separated renal tubules. Am J Physiol Renal Physiol 207:327–330
Caldovic L, Morizono H, Yu X, Thompson M, Shi D, Gallegos R, Allewell NM, Malamy MH, Tuchman M (2002) Identification, cloning and expression of the mouse N-acetylglutamate synthase gene. Biochem J 364(Pt 3):825–831
Cohen PP, Hayano M (1946) The conversion of citrulline to arginine (transimination) by tissue slices and homogenates. J Biol Chem 166(1):239–250
Coleman CS, Hu G, Pegg AE (2004) Putrescine biosynthesis in mammalian tissues. Biochem J 379(3):849–855
Conconi F, Grazi E (1965) Transamidinase of hog kidney. I. Purification and properties. J Biol Chem 240:2461–2464
Cremades A, Ruzafa C, Monserrat F, Lopez-Contreras AJ, Penafiel R (2004) Influence of dietary arginine on the anabolic effects of androgens. J Endocrinol 183(2):343–351
Crenn P, Vahedi K, Lavergne-Slove A, Cynober L, Matuchansky C, Messing B (2003) Plasma citrulline: a marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology 124(5):1210–1219
Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Benazeth S, Cynober L (2005) Almost all about citrulline in mammals. Amino Acids 29(3):177–205
Curis E, Crenn P, Cynober L (2007) Citrulline and the gut. Curr Opin Clin Nutr Metab Care 10(5):620–626
David RJ, Reddy SRR (1986) Arginine:glycine amidinotransferase. A comparative study in lizard and mouse tissues. Arch Int Physiol Biochim 94(2):77–83
Dejong CHC, Welters CFM, Deutz NEP, Heineman E, Soeters PB (1998) Renal arginine metabolism in fasted rats with subacute short bowel syndrome. Clin Sci 95(4):409–418
Deshmukh DR, Rusk CD (1989) Effects of arginine-free diet on urea cycle enzymes in young and adult ferrets. Enzyme 41:168–174
Dhanakoti SN, Brosnan JT, Herzberg GR, Brosnan ME (1990) Renal arginine synthesis: studies in vitro and in vivo. Am J Physiol Renal Physiol 259:E437–E442
Dhanakoti SN, Brosnan ME, Herzberg GR, Brosnan JT (1992) Cellular and subcellular localization of enzymes of arginine metabolism in rat kidney. Biochem J 282(2):369–375
Dubnoff JW, Borsook H (1948) Alpha-aminoadipic acid in arginine formation. J Biol Chem 173(1):425
Dworkin LD, Brenner BM (2000) The renal circulations. In: Brenner BM (ed) The kidney, vol 1, 6th edn. W. B. Saunders Company, Philadelphia, pp 247–285
Featherston WR, Rogers QR, Freedland RA (1973) Relative importance of kidney and liver in synthesis of arginine by the rat. Am J Physiol Renal Physiol 224(1):127–129
Funahashi M, Kato H, Shiosaka S, Nakagawa H (1981) Formation of arginine and guanidinoacetic acid in the kidney in vivo. Their regulations with the liver and their regulation. J Biochem 89(5):1347–1356
Gekle M, Silbernagl S (1991) Basolateral uptake and tubular metabolism of l-citrulline in the isolated-perfused non-filtering kidney of the African clawed toad (Xenopus laevis). Pflügers Arch Eur J Physiol 419:492–498
Gotoh T, Sonoki T, Nagasaki A, Terada K, Takiguchi M, Mori M (1996) Molecular cloning of cDNA for nonhepatic mitochondrial arginase (arginase II) and comparison of its induction with nitric oxide synthase in a murine macrophage-like cell line. FEBS Lett 395(2–3):119–122
Goutal I, Fairand A, Husson A (1999) Expression of the genes of arginine-synthesizing enzymes in the rat during development. Biol Neonate 76:253–260
Gross MD, Eggen MA, Simon AM, Van Pilsum JF (1986) The purification and characterization of human kidney l-arginine:glycine amidinotransferase. Arch Biochem Biophys 251(2):747–755
Gross MD, Simon AM, Jenny RJ, Gray ED, McGuire DM, Van Pilsum JF (1988) Multiple forms of rat kidney l-arginine:glycine amidinotransferase. J Nutr 118(11):1403–1409
Guder W, Wiesner W, Stukowski B, Wieland O (1971) Metabolism of isolated kidney tubules. Oxygen consumption, gluconeogenesis and the effect of cyclic nucleotides in tubules from starved rats. Hoppe-Seyler’s Z Physiol Chem 352(10):1319–1328
Hallemeesch MM, Ten Have GA, Deutz NE (2001) Metabolic flux measurements across portal drained viscera, liver, kidney and hindquarter in mice. Lab Anim 35(1):101–110
Hoogenraad NJ, Sutherland TM, Howlett GJ (1979) Effect of the transition-state analogue, delta-N-(phosphonacetyl)-l-ornithine on citrulline synthesis in isolated rat-liver mitochondria and on urea synthesis in isolated rat hepatocytes. Eur J Biochem 100(1):309–315
Hoogenraad N, Totino N, Elmer H, Wraight C, Alewood P, Johns RB (1985) Inhibition of intestinal citrulline synthesis causes severe growth retardation in rats. Am J Physiol Gastrointest Liver Physiol 249(12):G792–G799
Hus-Citharel A, Morel F (1986) Coupling of metabolic CO2 production to ion transport in isolated rat thick ascending limbs and collecting tubules. Pflügers Arch Eur J Physiol 407:421–427
Hus-Citharel A, Levillain O, Morel F (1995) Site of arginine synthesis and urea production along the nephron of a rodent species, Meriones shawi. Pflügers Arch Eur J Physiol 429:485–493
Husson A, Brasse-Lagnel C, Fairand A, Renouf S, Lavoinne A (2003) Argininosuccinate synthetase from the urea cycle to the citrulline–NO cycle. Eur J Biochem 270(9):1887–1899
Iyer RK, Bando JM, Jenkinson CP, Vockley JG, Kim PS, Kern RM, Cederbaum SD, Grody WW (1998) Cloning and characterization of the mouse and rat type II arginase genes. Mol Genet Metab 63(3):168–175
Jenkinson CP, Grody WW, Cederbaum SD (1996) Comparative properties of arginases. Comp Biochem Physiol [B] Biochem Mol Biol 114(1):107–132
Kaissling B, Kriz W (1979) Structural analysis of the rabbit kidney. In: Advances in anatomy embryology and cell biology, vol 56. Springer, Berlin, pp 1–123
Kakimoto Y, Akazawa S (1970) Isolation and identification of N-G, N-G- and N-G, N′-G-dimethyl-arginine, N-epsilon-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-delta-hydroxylysine from human urine. J Biol Chem 245(21):5751–5758
Kettner A, Silbernagl S (1985) Renal handling of citrulline. In: Dzurik R, Lichardus B, Guder WG (eds) Kidney metabolism and function. Martinus Nijhoff Publishers, Dordrecht, pp 51–60
Kimball ME, Jacoby LB (1980) Purification and properties of argininosuccinate synthetase from normal and canavanine-resistant human lymphoblasts. Biochemistry 19(4):705–709
Kimoto M, Whitley GSJ, Tsuji H, Ogawa T (1995) Detection of NG, NG-dimethylarginine dimethylaminohydrolase in human tissues using a monoclonal antibody. J Biochem Tokyo 117:237–238
Kimoto M, Sasakawa T, Tsuji H, Miyatake S, Oka T, Nio N, Ogawa T (1997) Cloning and sequencing of cDNA encoding NG, NG-dimethylarginine dimethylaminohydrolase from rat kidney. Biochim Biophys Acta 1337(1):6–10
Kimoto M, Miyatake S, Sasagawa T, Yamashita H, Okita M, Oka T, Ogawa T, Tsuji H (1998) Purification, cDNA cloning and expression of human NG, NG-dimethylarginine dimethylaminohydrolase. Eur J Biochem 258(2):863–868
Kobayashi Y (1963) Determination of histidine decarboxylase activity by liquid scintillation counting of C-14-O2. Anal Biochem 5:284–290
Kochakian CD, Garber EE, Bartlett MN (1940) Effect of estrogen alone and in combination with testosterone on the body and organ weights and the arginase and phosphatases of the organs of the mouse. Am J Physiol 155(2):265–271
Kriz W, Bankir L (1988) A standard nomenclature for structures of the kidney. The Renal Commission of the International Union of Physiological Sciences (IUPS). Am J Physiol Renal Fluid Electrolyte Physiol 254(1 Pt 2):F1–F8
Le Bouffant F, Hus-Citharel A, Morel F (1984) Metabolic CO2 production by isolated single pieces of rat distal nephron segments. Pflugers Arch Eur J Physiol 401:346–353
Leiper JM, Maria JS, Chubb A, MacAllister RJ, Charles IG, Whitley GS, Vallance P (1999) Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem J 343(Pt 1):209–214
Levillain O, Wiesinger H (2011) Expression and localization of argininosuccinate synthetase and argininosuccinate lyase in the female and male rat kidneys. In: Jacobs NL (ed) Arginine amino acid. Nova Science Publishers, Inc., New York, pp 111–123
Levillain O, Hus-citharel A, Morel F, Bankir L (1989) Production of urea from arginine in pars recta and collecting duct of the rat kidney. Renal Physiol Biochem 12:302–312
Levillain O, Hus-citharel A, Morel F, Bankir L (1990) Localization of arginine synthesis along rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 259(28):F916–F923
Levillain O, Hus-citharel A, Morel F, Bankir L (1992) Localization of urea and ornithine production along mouse and rabbit nephrons: functional significance. Am J Physiol Renal Fluid Electrolyte Physiol 263(32):F878–F885
Levillain O, Hus-Citharel A, Morel F, Bankir L (1993) Arginine synthesis in mouse and rabbit nephron: localization and functional significance. Am J Physiol Renal Fluid Electrolyte Physiol 264(33):F1038–F1045
Levillain O, Parvy P, Hus-Citharel A (1996) Arginine metabolism in cat kidney. J Physiol London 491(2):471–477
Levillain O, Balvay S, Peyrol S (2005a) Localization and differential expression of arginase II in male and female mouse kidney. Pflügers Arch Eur J Physiol 449(5):491–503
Levillain O, Balvay S, Peyrol S (2005b) Mitochondrial expression of arginase II in male and female rat inner medullary collecting ducts. J Histochem Cytochem 53(4):533–541
Levillain O, Rabier D, Duclos B, Gaudreau P, Vinay P (2008) l-arginine metabolism in dog kidney and isolated nephron segments. Metabolism 57(1):9–23
Lortie MJ, Novotny WF, Peterson OW, Vallon V, Malvey K, Mendonca M, Satriano J, Insel P, Thomson SC, Blantz RC (1996) Agmatine, a bioactive metabolite of arginine—production, degradation, and functional effects in the kidney of the rat. J Clin Invest 97(2):413–420
Luiking YC, Hallemeesch MM, Vissers YL, Lamers WH, Deutz NE (2004) In vivo whole body and organ arginine metabolism during endotoxemia (sepsis) is dependent on mouse strain and gender. J Nutr 134(10):2768S–2774S
Magri E, Baldoni G, Grazi E (1975) On the biosynthesis of creatine. Intramitochondrial localization of transamidinase from rat kidney. FEBS Lett 55(1):91–93
Marescau B, Nagels G, Possemiers I, De Broe ME, Becaus I, Billiouw JM, Lornoy W, De Deyn PP (1997) Guanidino compounds in serum and urine of nondialyzed patients with chronic renal insufficiency. Metabolism 46(9):1024–1031
McGuire DM, Tormanen CD, Segal IS, Van Pilsum JF (1980) The effect of growth hormone and thyroxine on the amount of l-arginine:glycine amidinotransferase in kidneys of hypophysectomized rats. Purification and some properties of rat kidney transamidinase. J Biol Chem 255(3):1152–1159
McGuire DM, Gross MD, Elde P, Pilsum JFV (1986) Localization of l-arginine-glycine amidinotransferase protein in rat tissues by immunofluorescence microscopy. J Histochem Cytochem 34(4):429–435
Mistry SK, Greenfeld Z, Morris SM Jr, Baylis C (2002) The “intestinal-renal” arginine biosynthetic axis in the aging rat. Mech Ageing Dev 123(8):1159–1165
Miyanaka K, Gotoh T, Nagasaki A, Takeya M, Ozaki M, Iwase K, Takiguchi M, Iyama K-I, Tomita K, Mori M (1998) Immunohistochemical localization of arginase II and other enzymes of arginine metabolism in rat kidney and liver. Histochem J 30(10):741–751
Mizutani A (1968) Cytochemical demonstration of ornithine carbamoyltransferase activity in liver mitochondria of rat and mouse. J Histochem Cytochem 16(3):172–180
Moncada S, Palmer RMJ, Higgs EA (1989) Biosynthesis of nitric oxide from l-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol 38:1709–1715
Morel F, Hus-Citharel A, Levillain O (1996) Biochemical heterogeneity of arginine metabolism along kidney proximal tubules. Kidney Int 49(6):1608–1610
Mori M, Aoyagi K, Tatibana M, Ishikawa T, Ishii H (1977) N delta-(phosphonacetyl)-l-ornithine, a potent transition state analogue inhibitor of ornithine carbamoyltransferase. Biochem Biophys Res Commun 76(3):900–904
Morris JG, Rogers QR (1978) Arginine: an essential amino acid for the cat. J Nutr 108:1944–1953
Morris JG, Rogers QR, Winterrowd DL, Kamikawa EM (1979) The utilisation of ornithine and citrulline by the growing kitten. J Nutr 109:724–729
Morris SM Jr, Moncman CL Jr, Holub JS, Hod Y (1989) Nutritional and hormonal regulation of mRNA abundance for arginine biosynthetic enzymes in kidney. Arch Biochem Biophys 273(1):230–237
Morris SM Jr, Sweeney WE Jr, Kepka DM Jr, O’brien WE, Avner ED (1991) Localization of arginine biosynthetic enzymes in renal proximal tubules and abundance of mRNA during development. Ped Res 25:151–154
Morris SM Jr, Bhamidipati D, Kepka-Lenhart D (1997) Human type II arginase: sequence analysis and tissue-specific expression. Gene 193(2):157–161
Morrissey JJ, McCraken R, Kaneto H, Vehaskari M, Montani D, Klahr S (1994) Location of an inducible nitric oxide synthase mRNA in the normal kidney. Kidney Int 45:998–1005
Morrissey J, McCracken R, Ishidoya S, Klahr S (1995) Partial cloning and characterization of an arginine decarboxylase in the kidney. Kidney Int 47(5):1458–1461
Natesan S, Reddy SR (2001) Compensatory changes in enzymes of arginine metabolism during renal hypertrophy in mice. Comp Biochem Physiol [B], Biochem Mol Biol 130(4):585–595
Ogawa T, Kimoto M, Sasaoka K (1987a) Occurrence of a new enzyme catalyzing the direct conversion of NG, NG-dimethyl-l-arginine to l-citrulline in rats. Biochem Biophys Res Commun 148(2):671–677
Ogawa T, Kimoto M, Wtanabe H, Sasaoka K (1987b) Metabolism of N G, N G- and N G, N′G dimethylarginine dimethylaminohydrolase in rats. Arch Biochem Biophys 252(2):526–537
Ogawa T, Kimoto M, Sasaoka K (1989) Purification and properties of a new enzyme, N G, N G-dimethylarginine dimethylaminohydrolase, from rat kidney. J Biol Chem 264(17):10205–10209
Onozato ML, Tojo A, Leiper J, Fujita T, Palm F, Wilcox CS (2008) Expression of NG, NG-dimethylarginine dimethylaminohydrolase and protein arginine N-methyltransferase isoforms in diabetic rat kidney: effects of angiotensin II receptor blockers. Diabetes 57(1):172–180
Osowska S, Moinard C, Neveux N, Loi C, Cynober L (2004) Citrulline increases arginine pools and restores nitrogen balance after massive intestinal resection. Gut 53(12):1781–1786
Palm F, Onozato ML, Luo Z, Wilcox CS (2007) Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol 293(6):H3227–H3245
Perez GO, Epstien M, Rietberg B, Loutzenhiser R (1978) Metabolism of arginine by the isolated perfused rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 235(4):F376–F380
Petrack B, Ratner S (1958) Biosynthesis of urea. VII. Reversible formation of argininosuccinic acid. J Biol Chem 233(6):1494–1500
Prins HA, Nijveldt RJ, Gasselt DV, Van Kemenade F, Teerlink T, Van Lambalgen AA, Rauwerda JA, Van Leeuwen PA (2002) The flux of arginine after ischemia-reperfusion in the rat kidney. Kidney Int 62(1):86–93
Rabinowitz L, Gunther RA, Shoji ES, Freedland RA, Avery EH (1973) Effects of high and low protein diets on sheep renal function and metabolism. Kidney Int 4:188–207
Ratner S (1973) Enzymes of arginine and urea synthesis. Adv Enzymol Relat Areas Mol Biol 39:1–90
Ratner S (1976) Enzymes of arginine and urea synthesis. In: Grisolia S, Baguena R, Mayor F (eds) The urea cycle. Wiley, New York, pp 181–219
Ratner S, Murakami-Murofushi K (1980) A new radiochemical assay for argininosuccinase with purified [14C]argininosuccinate. Anal Biochem 106(1):134–147
Ratner S, Pappas A (1949) Biosynthesis of urea I. Enzymatic mechanism of arginine synthesis from citrulline. J Biol Chem 179(3):1183–1198
Ratner S, Petrack B (1953) The mechanism of arginine synthesis from citrulline in kidney. J Biol Chem 200:175–185
Ratner S, Anslow WP Jr, Petrack B (1953a) Biosynthesis of urea. VI. Enzymatic cleavage of argininosuccinic acid to arginine and fumaric acid. J Biol Chem 204(1):115–125
Ratner S, Petrack B, Rochovansky O (1953b) Biosynthesis of urea. V. Isolation and properties of argininosuccinic acid. J Biol Chem 204(1):95–113
Robinson RR, Schmidt-Nielsen B (1963) Distribution of arginase within the kidneys of several vertebrate species. J Cell Physiol 62:147–157
Rochovansky O, Ratner S (1967) Biosynthesis of urea. XII. Further studies on argininosuccinate synthetase: substrate affinity and mechanism of action. J Biol Chem 242(17):3839–3849
Rogers QR, Phang JM (1985) Deficiency of pyrroline-5-carboxylate synthase in the intestinal mucosa of the cat. J Nutr 115:146–150
Schmidlin A, Kalbacher H, Wiesinger H (1997) Presence of argininosuccinate synthetase in glial cells as revealed by peptide-specific antisera. Biol Chem 378(1):47–50
Schuegraf A, Ratner S, Warner RC (1960) Free energy changes of the argininosuccinate synthetase reaction and of the hydrolysis of the inner pyrophosphate bond of adenosine triphosphate. J Biol Chem 235:3597–3602
Shi O, Kepka-Lenhart D, Morris SM Jr, O’Brien WE (1998) Structure of the murine arginase II gene. Mamm Genome 9(10):822–824
Shimogiri T, Bosak N, Morisson M, Okamoto S, Kawabe K, Maeda Y, Vignal A, Yasue H (2004) Assignment of CPS1, OTC, CRYD2, ARG2 and ASS genes to the chicken RH map. Genet Sel Evol 36(5):593–599
Skrzypek-Osiecka I, Robin Y, Porembska Z (1983) Purification of rat kidney arginases A1 and A4 and their subcellular distribution. Acta Biochim Pol 30(1):83–92
Soulet D, Baverel G, Levillain O (1997) Localisation de l’ornithine décarboxylase le long du néphron de souris. Néphrologie 18(4):154 (abstract)
Spector EB, Jenkinson CP, Grigor MR, Kern RM, Cederbaum SD (1994) Subcellular location and differential antibody specificity of arginase in tissue culture and whole animals. Int J Dev Neurosci 12(4):337–342
Szepesi B, Avery EH, Freedland RA (1970) Role of kidney in gluconeogenesis and amino acid catabolism. Am J Physiol Renal Physiol 219:1627–1631
Takeda M, Koide H, Jung KY, Endou H (1992) Intranephron distribution of glycine-amidinotransferase activity in rats. Renal Physiol Biochem 15:113–118
Tojo A, Welch WJ, Bremer V, Kimoto M, Kimura K, Omata M, Ogawa T, Vallance P, Wilcox CS (1997) Colocalization of dimethylating enzymes and NOS functional effects of methylarginines in rat kidney. Kidney Int 52:1593–1601
Tormanen CD, Sutter BE (1985) Changes in kidney transamidinase activity during development in male and female rats. Biosci Rep 5(4):309–314
Tran CT, Leiper JM, Vallance P (2003) The DDAH/ADMA/NOS pathway. Atheroscler Suppl 4(4):33–40
Vadgama JV, Evered DF (1992) Characteristics of l-citrulline transport across rat small intestine in vitro. Pediatr Res 32(4):472–478
Van Pilsum JF, Stephens GC, Taylor D (1972) Distribution of creatine, guanidinoacetate and the enzymes for their biosynthesis in the animal kingdom. Implications for phylogeny. Biochem J 126(2):325–345
Vinay P, Gougoux A, Lemieux G (1981) Isolation of a pure suspension of rat proximal tubules. Am J Physiol Renal Physiol 241(4):F403–F411
Wakabayashi Y, Yamada E, Yoshida T, Takahashi N (1995) Effect of intestinal resection and arginine-free diet on rat physiology. Am J Physiol Gastrointest Liver Physiol 269(32):G313–G318
Wakui H, Komatsuda A, Itoh H, Kobayashi R, Nakamoto Y, Miura AB (1992) Renal argininosuccinate synthetase: purification, immunohistochemical localization, and elastin-binding property. Renal Physiol Biochem 15(1):1–9
Windmueller HG, Spaeth AE (1974) Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem 249:5070–5079
Windmueller HG, Spaeth AE (1975) Intestinal metabolism of glutamime and glutamate from the lumen as compared to glutamine from blood. Arch Biochem Biophys 171:662–672
Windmueller HG, Spaeth AE (1978) Identification of ketone bodies and glutamine as the major respiratory fuels in vivo for post absorptive rat small intestin. J Biol Chem 253(1):69–76
Windmueller HG, Spaeth AE (1980) Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. J Biol Chem 255:107–112
Windmueller HG, Spaeth AE (1981) Source and fate of circulating citrulline. Am J Physiol Renal Physiol 241:E473–E480
Witzmann FA, Fultz CD, Grant RA, Wright LS, Kornguth SE, Siegel FL (1998) Differential expression of cytosolic proteins in the rat kidney cortex and medulla: preliminary proteomics. Electrophoresis 19(14):2491–2497
Wu G, Flynn NE, Yan W, Barstow DG Jr (1995) Glutamine metabolism in chick enterocytes: absence of pyrroline-5-carboxylase synthase and citrulline synthesis. Biochem J 306:717–721
Yu Y, Terada K, Nagasaki A, Takiguchi M, Mori M (1995) Preparation of recombinant argininosuccinate synthetase and argininosuccinate lyase: expression of the enzymes in rat tissues. J Biochem 117(25):952–957
Yu YM, Burke J, Tompkins RG, Martin R, Young VR (1996) Quantitative aspects of interorgan relationships among arginine and citrulline metabolism. Am J Physiol Endocrinol Metab 34(6):E1098–E1109
Yu H, Yoo PK, Aguirre CC, Tsoa RW, Kern RM, Grody WW, Cederbaum SD, Iyer RK (2003) Widespread expression of arginase I in mouse tissues. Biochemical and physiological implications. J Histochem Cytochem 51(9):1151–1160
Acknowledgments
The author is indebted to Dr Ljubica Caldovic and Dr Hiroki Morizono, Washington DC, USA for reading and improving the paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Levillain, O. Expression and function of arginine-producing and consuming-enzymes in the kidney. Amino Acids 42, 1237–1252 (2012). https://doi.org/10.1007/s00726-011-0897-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-0897-z