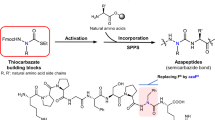
Abstract
Angiotensin-(1–7) [Ang-(1–7)], a heptapeptide hormone of the renin–angiotensin–aldosterone system, is a promising candidate as a treatment for cancer that reflects its anti-proliferative and anti-angiogenic properties. However, the peptide’s therapeutic potential is limited by the short half-life and low bioavailability resulting from rapid enzymatic metabolism by peptidases including angiotensin-converting enzyme (ACE) and dipeptidyl peptidase 3 (DPP 3). We report the facile assembly of three novel Ang-(1–7) analogues by solid-phase peptide synthesis which incorporates the cyclic non-natural δ-amino acid ACCA. The analogues containing the ACCA substitution at the site of ACE cleavage exhibit complete resistance to human ACE, while substitution at the DDP 3 cleavage site provided stability against DPP 3 hydrolysis. Furthermore, the analogues retain the anti-proliferative properties of Ang-(1–7) against the 4T1 and HT-1080 cancer cell lines. These results suggest that ACCA-substituted Ang-(1–7) analogues which show resistance against proteolytic degradation by peptidases known to hydrolyze the native heptapeptide may be novel therapeutics in the treatment of cancer.








Similar content being viewed by others
Abbreviations
- ACCA:
-
cis-3-(Aminomethyl)cyclobutanecarboxylic acid
- ACE:
-
Angiotensin-converting enzyme
- HATU:
-
N-[(Dimethylamino)-1H-1,2,3-triazolo-[4,5-b]pyridin-1-ylmethylene]-N-methylmethanaminium hexafluorophosphate N-oxide
- HEPES:
-
4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid
- HPLC:
-
High-performance liquid chromatography
- HRMS:
-
High-resolution mass spectrometry
- 2CT resin:
-
2-Chlorotrityl resin
- NMP:
-
N-methylpyrrolidone
- DIPEA:
-
N,N-Diisopropylethylamine
- DPP 3:
-
Dipeptidyl peptidase 3
- Fmoc:
-
9-Fluorenylmethoxycarbonyl
- Fmoc-OSu:
-
N-(9-Fluorenylmethoxycarbonyloxy)succinimide
- Pbf:
-
2,2,4,6,7-Pentamethyldihydrobenzyofuran-5-sulfonyl
- t-Bu:
-
t-Butyl
- Trt:
-
Trityl
- TFA:
-
Trifluoroacetic acid
- SPPS:
-
Solid-phase peptide synthesis
References
Chappell MC (2016) Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am J Physiol Heart Circ Physiol 310:H137–H152
Chappell MC, Pirro NT, Sykes A, Ferrario CM (1998) Metabolism of angiotensin-(1–7) by angiotensin-converting enzyme. Hypertension 31:362–367
Cook KL, Metheny-Barlow LJ, Tallant EA, Gallagher PE (2010) Angiotensin-(1–7) reduces fibrosis in orthotopic breast tumors. Cancer Res 70:8319–8328
Cruz-Diaz N, Wilson BA, Pirro NT, Brosnihan KB, Marshall AC, Chappell MC (2016) Identification of dipeptidyl peptidase 3 as the angiotensin-(1–7) degrading peptidase in human HK-2 renal epithelial cells. Peptides 83:29–37
Fraga-Silva RA, Costa-Fraga FP, De Sousa FB, Alenina N, Bader M, Sinisterra RD, Santos RA (2011) An orally active formulation of angiotensin-(1–7) produces an antithrombotic effect. Clinics 66:837–841
Gallagher PE, Tallant EA (2004) Inhibition of human lung cancer cell growth by angiotensin-(1–7). Carcingenesis 25:2045–2052
Gallagher PE, Arter AL, Deng G, Tallant EA (2014) Angiotensin-(1–7): a peptide hormone with anti-cancer activity. Curr Med Chem 21:2417–2423
Kluskens LD, Nelemans SA, Rink R, de Vries L, Meter-Arkema A, Wang Y, Walther T, Kuipers A, Moll GN, Haas M (2009) Angiotensin-(1–7) with thioether bridge: an angiotensin-converting enzyme-resistant, potent angiotensin-(1–7) analog. J Pharmacol Exp Ther 328:849–854
Krishnan B, Smith TL, Dubey P, Zapadka ME, Torti FM, Willingham MC, Tallant EA, Gallagher PE (2013a) Angiotensin-(1–7) attenuates metastatic prostate cancer and reduces osteoclastogenesis. Prostate 73:71–82
Krishnan B, Torti FM, Gallagher PE, Tallant EA (2013b) Angiotensin-(1–7) reduces proliferation and angiogenesis of human prostate cancer xenografts with a decrease in angiogenic factors and an increase in sFlt-1. Prostate 73:60–70
Lula I, Denadai ÂL, Resende JM, de Sousa FB, de Lima GF, Pilo-Veloso D, Heine T, Duarte HA, Santos RAS, Sinisterra RD (2007) Study of angiotensin-(1–7) vasoactive peptide and its β-cyclodextrin inclusion complexes: complete sequence-specific NMR assignments and structural studies. Peptides 28:2199–2210
Marshall AC, Pirro NT, Rose JC, Diz DI, Chappell MC (2014a) Evidence for an angiotensin-(1–7) neuropeptidase expressed in the brain medulla and CSF of sheep. J Neurochem 130:313–323
Marshall AC, Shaltout HA, Pirro NT, Rose JC, Diz DI, Chappell MC (2014b) Enhanced activity of an angiotensin-(1–7) neuropeptidase in glucocorticoid-induced fetal programming. Peptides 52:74–81
Menon J, Soto-Pantoja DR, Callahan MF, Cline JM, Ferrario CM, Tallant EA, Gallagher PE (2007) Angiotensin-(1–7) inhibits growth of human lung adenocarcinoma xenografts in nude mice through a reduction in cyclooxygenase-2. Cancer Res 67:2809–2815
Ni L, Feng Y, Wan H, Ma Q, Fan L, Qian Y, Li Q, Xiang Y, Gao B (2012) Angiotensin-(1–7) inhibits the migration and invasion of A549 human lung adenocarcinoma cells through inactivation of the PI3K/Akt and MAPK signaling pathways. Oncol Rep 27:783–790
O’Reilly E, Pes L, Ortin Y, Müller-Bunz H, Paradisi F (2013) Synthesis of a conformationally constrained δ-amino acid building block. Amino Acids 44:511–518
Paryzek Z, Koenig H, Tabaczka B (2003) Ammonium formate/palladium on carbon: a versatile system for catalytic hydrogen transfer reductions of carbon-carbon double bonds. Synthesis 2003:2023–2026
Pes L (2013) Study of the non proteinogenic delta-amino acid ACCA, its biological investigation and application. PhD thesis, University College Dublin
Petty WJ, Miller AA, McCoy TP, Gallagher PE, Tallant EA, Torti FM (2009) Phase I and pharmacokinetic study of angiotensin-(1–7), an endogenous antiangiogenic hormone. Clin Cancer Res 15:7398–7404
Petty WJ, Aklilu M, Varela VA, Lovato J, Savage PD, Miller AA (2012) Reverse translation of phase I biomarker findings links the activity of angiotensin-(1–7) to repression of hypoxia inducible factor-1alpha in vascular sarcomas. BMC Cancer 12:404
Pham H, Schwartz BM, Delmore JE, Reed E, Cruickshank S, Drummond L, Rodgers KE, Peterson KJ, di Zerega GS (2013) Pharmacodynamic stimulation of thrombogenesis by angiotensin (1–7) in recurrent ovarian cancer patients receiving gemcitabine and platinum-based chemotherapy. Cancer Chemother Pharmacol 71:965–972
Rodgers KE, Oliver J, di Zerega GS (2006) Phase I/II dose escalation study of angiotensin 1–7 [A(1–7)] administered before and after chemotherapy in patients with newly diagnosed breast cancer. Cancer Chemother Pharmacol 57:559–568
Santos RAS, Silva ACS, Maric C, Silva DMR, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SVB, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss H-P, Speth R, Walther T (2003) Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A 100:8258–8263
Silva-Barcellos NM, Frézard F, Caligiorne S, Santos RAS (2001) Long-lasting cardiovascular effects of liposome-entrapped angiotensin-(1–7) at the rostral ventrolateral medulla. Hypertension 38:1266–1271
Soto-Pantoja DR, Menon J, Gallagher PE, Tallant EA (2009) Angiotensin-(1–7) inhibits tumor angiogenesis in human lung cancer xenografts with a reduction in vascular endothelial growth factor. Mol Cancer Ther 8:1676–1683
Acknowledgements
We would like to thank the National University of Ireland for funding this research. We would like to acknowledge the facilities in the Centre for Synthesis and Chemical Biology (CSCB), funded by the Higher Education Authority’s Programme for Research in Third Level Institutions (PRTLIs). We are grateful to Dr. Helge Müller-Bunz for his work on the X-ray crystal structure. We would also like to acknowledge the Science Foundation Ireland Equipment Grant 06/RFP/CHO024/EC07, the National Institutes of Health (NIH) HD084227, the American Heart Association Grants AHA-151521 and AHA-355741, the Randi B. Weiss Cancer Research Fund (Winston-Salem, NC), and the Farley-Hudson Foundation (Jacksonville, NC). Finally, we acknowledge the technical expertise of Nancy T. Pirro for the peptide metabolism studies and L. Tenille Howard for cell proliferation studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: T. Langer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wester, A., Devocelle, M., Tallant, E.A. et al. Stabilization of Angiotensin-(1–7) by key substitution with a cyclic non-natural amino acid. Amino Acids 49, 1733–1742 (2017). https://doi.org/10.1007/s00726-017-2471-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-017-2471-9