Abstract
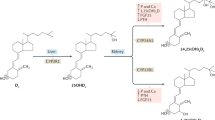
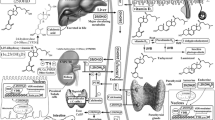
The keratinocytes of the skin are unique in being not only the primary source of vitamin D for the body, but also possessing the enzymatic machinery to metabolize vitamin D to active metabolites [in particular, 1,25 dihydroxyvitamin D (1,25(OH)2D)] and the vitamin D receptor (VDR) that enables the keratinocytes to respond to the 1,25(OH)2D they produce. Numerous functions of the skin are regulated by vitamin D and/or its receptor: these include inhibition of proliferation, stimulation of differentiation including formation of the permeability barrier, promotion of innate immunity, regulation of the hair follicle cycle, and suppression of tumor formation. Regulation of these actions is exerted by a number of different coregulators including the coactivators DRIP and SRC, a less well known inhibitor, hairless, and β-catenin. Different coregulators appear to be involved in different VDR-regulated functions. This review examines the various functions of vitamin D and its receptor, and to the extent known explores the mechanisms by which these functions are regulated.





Similar content being viewed by others
References
Bikle DD, Pillai S (1993) Vitamin D, calcium, and epidermal differentiation. Endocr Rev 14:3–19
Pillai S, Bikle DD (1991) Role of intracellular-free calcium in the cornified envelope formation of keratinocytes: differences in the mode of action of extracellular calcium and 1,25 dihydroxyvitamin D3. J Cell Physiol 146:94–100
Bikle DD, Pillai S, Gee E (1991) Squamous carcinoma cell lines produce 1,25 dihydroxyvitamin D, but fail to respond to its prodifferentiating effect. J Invest Dermatol 97:435–441
Hosomi J, Hosoi J, Abe E, Suda T, Kuroki T (1983) Regulation of terminal differentiation of cultured mouse epidermal cells by 1-alpha, 25-dihydroxyvitamin D3. Endocrinology 113:1950–1957
Smith EL, Walworth NC, Holick MF (1986) Effect of 1-alpha, 25-dihydroxyvitamin D3 on the morphologic and biochemical differentiation of cultured human epidermal keratinocytes grown in serum-free conditions. J Invest Dermatol 86:709–714
McLane JA, Katz M, Abdelkader N (1990) Effect of 1,25-dihydroxyvitamin D3 on human keratinocytes grown under different culture conditions. In Vitro Cell Dev Biol 26:379–387
Hawker NP, Pennypacker SD, Chang SM, Bikle DD (2007) Regulation of human epidermal keratinocyte differentiation by the vitamin D receptor and its coactivators DRIP205, SRC2, and SRC3. J Invest Dermatol 127:874
Matsumoto K, Hashimoto K, Nishida Y, Hashiro M, Yoshikawa K (1990) Growth-inhibitory effects of 1,25-dihydroxyvitamin D3 on normal human keratinocytes cultured in serum-free medium. Biochem Biophys Res Commun 166:916–923
Oda Y, Uchida Y, Moradian S, Crumrine D, Elias PM, Bikle DD (2009) Vitamin D receptor and coactivators SRC2 and 3 regulate epidermis-specific sphingolipid production and permeability barrier formation. J Invest Dermatol 129:1367–1378
Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U, Bikle DD, Modlin RL, Gallo RL (2007) Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest 117:803–811
Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL (2006) Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology 118:509–519
Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB (1997) Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA 94:9831–9835
Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, Dardenne O, Xie Z, Arnaud RS, Feingold K, Elias PM (2004) 25 Hydroxyvitamin D 1-alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol 122:984–992
Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC, Leary C, Chang S, Crumrine D, Yoshizawa T, Kato S, Bikle DD (2002) Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol 118:11–16
Bikle DD, Elalieh H, Chang S, Xie Z, Sundberg JP (2006) Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol 207:340–353
Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M (2001) Extrarenal expression of 25-hydroxyvitamin D(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab 86:888–894
Milde P, Hauser U, Simon T, Mall G, Ernst V, Haussler MR, Frosch P, Rauterberg EW (1991) Expression of 1,25-dihydroxyvitamin D3 receptors in normal and psoriatic skin. J Invest Dermatol 97:230–239
Stumpf WE, Clark SA, Sar M, DeLuca HF (1984) Topographical and developmental studies on target sites of 1,25 (OH)2 vitamin D3 in skin. Cell Tissue Res 238:489–496
Oda Y, Sihlbom C, Chalkley RJ, Huang L, Rachez C, Chang CP, Burlingame AL, Freedman LP, Bikle DD (2003) Two distinct coactivators, DRIP/mediator and SRC/p160, are differentially involved in vitamin D receptor transactivation during keratinocyte differentiation. Mol Endocrinol 17:2329–2339
McKenna NJ, Lanz RB, O’Malley BW (1999) Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20:321–344
Oda Y, Ishikawa MH, Hawker NP, Yun QC, Bikle DD (2007) Differential role of two VDR coactivators, DRIP205 and SRC-3, in keratinocyte proliferation and differentiation. J Steroid Biochem Mol Biol 103:776–780
Schauber J, Oda Y, Buchau AS, Steinmeyer A, Zugel U, Bikle DD, Gallo RL (2008) Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol 128:816–824
Rachez C, Lemon BD, Suldan Z, Bromleigh V, Gamble M, Naar AM, Erdjument-Bromage H, Tempst P, Freedman LP (1999) Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature (Lond) 398:824–828
Rachez C, Gamble M, Chang CP, Atkins GB, Lazar MA, Freedman LP (2000) The DRIP complex and SRC-1/p160 coactivators share similar nuclear receptor binding determinants but constitute functionally distinct complexes. Mol Cell Biol 20:2718–2726
Leo C, Chen JD (2000) The SRC family of nuclear receptor coactivators. Gene (Amst) 245:1–11
Teichert A, Arnold LA, Otieno S, Oda Y, Augustinaite I, Geistlinger TR, Kriwacki RW, Guy RK, Bikle DD (2009) Quantification of the vitamin D receptor-coregulator interaction. Biochemistry 48:1454–1461
Acevedo ML, Lee KC, Stender JD, Katzenellenbogen BS, Kraus WL (2004) Selective recognition of distinct classes of coactivators by a ligand-inducible activation domain. Mol Cell 13:725–738
Christakos S, Dhawan P, Liu Y, Peng X, Porta A (2003) New insights into the mechanisms of vitamin D action. J Cell Biochem 88:695–705
Rachez C, Freedman LP (2000) Mechanisms of gene regulation by vitamin D(3) receptor: a network of coactivator interactions. Gene (Amst) 246:9–21
Carvallo L, Henriquez B, Paredes R, Olate J, Onate S, Van Wijnen AJ, Lian JB, Stein G, Stein JL, Montecino M (2008) 1,25-Dihydroxy vitamin D3-enhanced expression of the osteocalcin gene involves increased promoter occupancy of basal transcription regulators and gradual recruitment of the 1,25-dihydroxy vitamin D3 receptor-SRC-1 coactivator complex. J Cell Physiol 214:740–749
Issa LL, Leong GM, Sutherland RL, Eisman JA (2002) Vitamin D analogue-specific recruitment of vitamin D receptor coactivators. J Bone Miner Res 17:879–890
Bouillon R, Verlinden L, Eelen G, De Clercq PV M, Mathieu C, Verstuyf A (2005) Mechanisms for the selective action of vitamin D analogs. J Steroid Biochem Mol Biol 97:21–30
Maeda Y, Rachez C, Hawel IL, Byus CV, Freedman LP, Sladek FM (2002) Polyamines modulate the interaction between nuclear receptors and vitamin D receptor-interacting protein 205. Mol Endocrinol 16:1502–1510
Peleg S, Ismail A, Uskokovic M, Avnur Z (2003) Evidence for tissue- and cell-type selective activation of the vitamin D receptor by Ro-26–9228, a noncalcemic analog of vitamin D3. J Cell Biochem 88:267–273
Shimizu H, Morgan BA (2004) Wnt signaling through the beta-catenin pathway is sufficient to maintain, but not restore, anagen-phase characteristics of dermal papilla cells. J Invest Dermatol 122:239–245
Kishimoto J, Burgeson RE, Morgan BA (2000) Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev 14:1181–1185
Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G (2004) Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22:411–417
Langbein L, Rogers MA, Praetzel S, Winter H, Schweizer J (2003) K6irs1, K6irs2, K6irs3, and K6irs4 represent the inner-root-sheath-specific type II epithelial keratins of the human hair follicle. J Invest Dermatol 120:512–522
Zhou P, Byrne C, Jacobs J, Fuchs E (1995) Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev 9:700–713
Hochberg Z, Gilhar A, Haim S, Friedman-Birnbaum R, Levy J, Benderly A (1985) Calcitriol-resistant rickets with alopecia. Arch Dermatol 121:646–647
Marx SJ, Bliziotes MM, Nanes M (1986) Analysis of the relation between alopecia and resistance to 1,25-dihydroxyvitamin D. Clin Endocrinol (Oxf) 25:373–381
Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S (1997) Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 16:391–396
Sakai Y, Demay MB (2000) Evaluation of keratinocyte proliferation and differentiation in vitamin D receptor knockout mice. Endocrinology 141:2043–2049
Kong J, Li XJ, Gavin D, Jiang Y, Li YC (2002) Targeted expression of human vitamin d receptor in the skin promotes the initiation of the postnatal hair follicle cycle and rescues the alopecia in vitamin D receptor null mice. J Invest Dermatol 118:631–638
Chen CH, Sakai Y, Demay MB (2001) Targeting expression of the human vitamin D receptor to the keratinocytes of vitamin D receptor null mice prevents alopecia. Endocrinology 142:5386–5389
Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, Delling G, Demay MB (1998) Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology 139:4391–4396
Panteleyev AA, Botchkareva NV, Sundberg JP, Christiano AM, Paus R (1999) The role of the hairless (hr) gene in the regulation of hair follicle catagen transformation. Am J Pathol 155:159–171
Miller J, Djabali K, Chen T, Liu Y, Ioffreda M, Lyle S, Christiano AM, Holick M, Cotsarelis G (2001) Atrichia caused by mutations in the vitamin D receptor gene is a phenocopy of generalized atrichia caused by mutations in the hairless gene. J Invest Dermatol 117:612–617
Ahmad W, Faiyaz ul Haque M, Brancolini V, Tsou HC, ul Haque S, Lam H, Aita VM, Owen J, deBlaquiere M, Frank J, Cserhalmi-Friedman PB, Leask A, McGrath JA, Peacocke M, Ahmad M, Ott J, Christiano AM (1998) Alopecia universalis associated with a mutation in the human hairless gene. Science 279:720–724
Millar SE (2002) Molecular mechanisms regulating hair follicle development. J Invest Dermatol 118:216–225
Stenn KS, Paus R (2001) Controls of hair follicle cycling. Physiol Rev 81:449–494
Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W (2001) beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105:533–545
DasGupta R, Rhee H, Fuchs E (2002) A developmental conundrum: a stabilized form of beta-catenin lacking the transcriptional activation domain triggers features of hair cell fate in epidermal cells and epidermal cell fate in hair follicle cells. J Cell Biol 158:331–344
Zarach JM, Beaudoin GM III, Coulombe PA, Thompson CC (2004) The co-repressor hairless has a role in epithelial cell differentiation in the skin. Development (Camb) 131:4189–4200
Chiang C, Swan RZ, Grachtchouk M, Bolinger M, Litingtung Y, Robertson EK, Cooper MK, Gaffield W, Westphal H, Beachy PA, Dlugosz AA (1999) Essential role for sonic hedgehog during hair follicle morphogenesis. Dev Biol 205:1–9
Beaudoin GM III, Sisk JM, Coulombe PA, Thompson CC (2005) Hairless triggers reactivation of hair growth by promoting Wnt signaling. Proc Natl Acad Sci USA 102:14653–14658
Reddy ST, Andl T, Lu MM, Morrisey EE, Millar SE (2004) Expression of Frizzled genes in developing and postnatal hair follicles. J Invest Dermatol 123:275–282
Shah S, Hecht A, Pestell R, Byers SW (2003) Trans-repression of beta-catenin activity by nuclear receptors. J Biol Chem 278:48137–48145
Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, Zinser G, Valrance M, Aranda A, Moras D, Norman A, Welsh J, Byers SW (2006) The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol Cell 21:799–809
Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Munoz A (2001) Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol 154:369–387
Djabali K, Aita VM, Christiano AM (2001) Hairless is translocated to the nucleus via a novel bipartite nuclear localization signal and is associated with the nuclear matrix. J Cell Sci 114:367–376
Thompson CC, Bottcher MC (1997) The product of a thyroid hormone-responsive gene interacts with thyroid hormone receptors. Proc Natl Acad Sci USA 94:8527–8532
Engelhard A, Christiano AM (2004) The hairless promoter is differentially regulated by thyroid hormone in keratinocytes and neuroblastoma cells. Exp Dermatol 13:257–264
Xie Z, Chang S, Oda Y, Bikle DD (2006) Hairless suppresses vitamin D receptor transactivation in human keratinocytes. Endocrinology 147:314–323
Hsieh JC, Sisk JM, Jurutka PW, Haussler CA, Slater SA, Haussler MR, Thompson CC (2003) Physical and functional interaction between the vitamin D receptor and hairless corepressor, two proteins required for hair cycling. J Biol Chem 278:38665–38674
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512
Xie Z, Bikle DD (2007) The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J Biol Chem 282:8695–8703
Bienz M (2005) beta-Catenin: a pivot between cell adhesion and Wnt signalling. Curr Biol 15:R64–R67
Chan EF, Gat U, McNiff JM, Fuchs E (1999) A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet 21:410–413
Gat U, DasGupta R, Degenstein L, Fuchs E (1998) De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 95:605–614
Xia J, Urabe K, Moroi Y, Koga T, Duan H, Li Y, Furue M (2006) beta-Catenin mutation and its nuclear localization are confirmed to be frequent causes of Wnt signaling pathway activation in pilomatricomas. J Dermatol Sci 41:67–75
Palmer HG, Anjos-Afonso F, Carmeliet G, Takeda H, Watt FM (2008) The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS ONE 3:e1483
Cianferotti L, Cox M, Skorija K, Demay MB (2007) Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc Natl Acad Sci USA 104:9428–9433
Greenlee RT, Hill-Harmon MB, Murray T, Thun M (2001) Cancer statistics, 2001. CA Cancer J Clin 51:15–36
Freeman SE, Hacham H, Gange RW, Maytum DJ, Sutherland JC, Sutherland BM (1989) Wavelength dependence of pyrimidine dimer formation in DNA of human skin irradiated in situ with ultraviolet light. Proc Natl Acad Sci USA 86:5605–5609
Hussein MR (2005) Ultraviolet radiation and skin cancer: molecular mechanisms. J Cutan Pathol 32:191–205
Daya-Grosjean L, Sarasin A (2005) The role of UV induced lesions in skin carcinogenesis: an overview of oncogene and tumor suppressor gene modifications in xeroderma pigmentosum skin tumors. Mutat Res 571:43–56
Ziegler A, Leffell DJ, Kunala S, Sharma HW, Gailani M, Simon JA, Halperin AJ, Baden HP, Shapiro PE, Bale AE, Brash DE (1993) Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers. Proc Natl Acad Sci USA 90:4216–4220
Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, Remington L, Jacks T, Brash DE (1994) Sunburn and p53 in the onset of skin cancer. Nature (Lond) 372:773–776
Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, Halperin AJ, Ponten J (1991) A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA 88:10124–10128
Bito T, Ueda M, Ahmed NU, Nagano T, Ichihashi M (1995) Cyclin D and retinoblastoma gene product expression in actinic keratosis and cutaneous squamous cell carcinoma in relation to p53 expression. J Cutan Pathol 22:427–434
Reifenberger J, Wolter M, Knobbe CB, Kohler B, Schonicke A, Scharwachter C, Kumar K, Blaschke B, Ruzicka T, Reifenberger G (2005) Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol 152:43–51
Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH Jr, Scott MP (1996) Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 272:1668–1671
Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden AB, Gillies S, Negus K, Smyth I, Pressman C, Leffell DJ, Gerrard B, Goldstein AM, Dean M, Toftgard R, Chenevix-Trench G, Wainwright B, Bale AE (1996) Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 85:841–851
Aszterbaum M, Rothman A, Johnson RL, Fisher M, Xie J, Bonifas JM, Zhang X, Scott MP, Epstein EH Jr (1998) Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol 110:885–888
Aszterbaum M, Epstein J, Oro A, Douglas V, LeBoit PE, Scott MP, Epstein EH Jr (1999) Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med 5:1285–1291
Ping XL, Ratner D, Zhang H, Wu XL, Zhang MJ, Chen FF, Silvers DN, Peacocke M, Tsou HC (2001) PTCH mutations in squamous cell carcinoma of the skin. J Invest Dermatol 116:614–616
Zinser GM, Sundberg JP, Welsh J (2002) Vitamin D(3) receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis (Oxf) 23:2103–2109
Indra AK, Castaneda E, Antal MC, Jiang M, Messaddeq N, Meng X, Loehr CV, Gariglio P, Kato S, Wahli W, Desvergne B, Metzger D, Chambon P (2007) Malignant transformation of DMBA/TPA-induced papillomas and nevi in the skin of mice selectively lacking retinoid-X-receptor alpha in epidermal keratinocytes. J Invest Dermatol 127:1250–1260
Ellison TI, Smith MK, Gilliam AC, Macdonald PN (2008) Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol 128:2508–2517
Regl G, Kasper M, Schnidar H, Eichberger T, Neill GW, Ikram MS, Quinn AG, Philpott MP, Frischauf AM, Aberger F (2004) The zinc-finger transcription factor GLI2 antagonizes contact inhibition and differentiation of human epidermal cells. Oncogene 23:1263–1274
Barnfield PC, Zhang X, Thanabalasingham V, Yoshida M, Hui CC (2005) Negative regulation of Gli1 and Gli2 activator function by Suppressor of fused through multiple mechanisms. Differentiation 73:397–405
Wakabayashi Y, Mao JH, Brown K, Girardi M, Balmain A (2007) Promotion of Hras-induced squamous carcinomas by a polymorphic variant of the Patched gene in FVB mice. Nature (Lond) 445:761–765
Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP (2006) Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol 4:e232
Svard J, Heby-Henricson K, Persson-Lek M, Rozell B, Lauth M, Bergstrom A, Ericson J, Toftgard R, Teglund S (2006) Genetic elimination of suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell 10:187–197
Regl G, Kasper M, Schnidar H, Eichberger T, Neill GW, Philpott MP, Esterbauer H, Hauser-Kronberger C, Frischauf AM, Aberger F (2004) Activation of the BCL2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Res 64:7724–7731
Regl G, Neill GW, Eichberger T, Kasper M, Ikram MS, Koller J, Hintner H, Quinn AG, Frischauf AM, Aberger F (2002) Human GLI2 and GLI1 are part of a positive feedback mechanism in basal cell carcinoma. Oncogene 21:5529–5539
Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC, Dlugosz AA (2000) Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet 24:216–217
Nilsson M, Unden AB, Krause D, Malmqwist U, Raza K, Zaphiropoulos PG, Toftgard R (2000) Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci USA 97:3438–3443
Oro AE, Higgins KM, Hu Z, Bonifas JM, Epstein EH Jr, Scott MP (1997) Basal cell carcinomas in mice overexpressing sonic hedgehog. Science 276:817–821
Fan H, Oro AE, Scott MP, Khavari PA (1997) Induction of basal cell carcinoma features in transgenic human skin expressing sonic hedgehog. Nat Med 3:788–792
Tojo M, Mori T, Kiyosawa H, Honma Y, Tanno Y, Kanazawa KY, Yokoya S, Kaneko F, Wanaka A (1999) Expression of sonic hedgehog signal transducers, patched and smoothened, in human basal cell carcinoma. Pathol Int 49:687–694
Bonifas JM, Pennypacker S, Chuang PT, McMahon AP, Williams M, Rosenthal A, De Sauvage FJ, Epstein EH Jr (2001) Activation of expression of hedgehog target genes in basal cell carcinomas. J Invest Dermatol 116:739–742
Eichberger T, Regl G, Ikram MS, Neill GW, Philpott MP, Aberger F, Frischauf AM (2004) FOXE1, a new transcriptional target of GLI2 is expressed in human epidermis and basal cell carcinoma. J Invest Dermatol 122:1180–1187
Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, Mader S, White JH (2005) Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol 19:2685–2695
Saldanha G, Ghura V, Potter L, Fletcher A (2004) Nuclear beta-catenin in basal cell carcinoma correlates with increased proliferation. Br J Dermatol 151:157–164
Iwatsuki K, Liu HX, Gronder A, Singer MA, Lane TF, Grosschedl R, Mistretta CM, Margolskee RF (2007) Wnt signaling interacts with Shh to regulate taste papilla development. Proc Natl Acad Sci USA 104:2253–2258
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bikle, D.D. Vitamin D and the skin. J Bone Miner Metab 28, 117–130 (2010). https://doi.org/10.1007/s00774-009-0153-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-009-0153-8