Abstract
Introduction
Osteoblasts require substantial amounts of energy to synthesize the bone matrix and coordinate skeleton mineralization. This study analyzed the effects of mitochondrial dysfunction on bone formation, nano-organization of collagen and apatite, and the resultant mechanical function in mouse limbs.
Materials and methods
Limb mesenchyme-specific Tfam knockout (Tfamf/f;Prx1-Cre: Tfam-cKO) mice were analyzed morphologically and histologically, and gene expressions in the limb bones were assessed by in situ hybridization, qPCR, and RNA sequencing (RNA-seq). Moreover, we analyzed the mitochondrial function of osteoblasts in Tfam-cKO mice using mitochondrial membrane potential assay and transmission electron microscopy (TEM). We investigated the pathogenesis of spontaneous bone fractures using immunohistochemical analysis, TEM, birefringence analyzer, microbeam X-ray diffractometer and nanoindentation.
Results
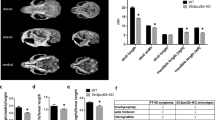
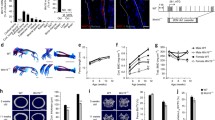
Forelimbs in Tfam-cKO mice were significantly shortened from birth, and spontaneous fractures occurred after birth, resulting in severe limb deformities. Histological and RNA-seq analyses showed that bone hypoplasia with a decrease in matrix mineralization was apparent, and the expression of type I collagen and osteocalcin was decreased in osteoblasts of Tfam-cKO mice, although Runx2 expression was unchanged. Decreased type I collagen deposition and mineralization in the matrix of limb bones in Tfam-cKO mice were associated with marked mitochondrial dysfunction. Tfam-cKO mice bone showed a significantly lower Young’s modulus and hardness due to poor apatite orientation which is resulted from decreased osteocalcin expression.
Conclusion
Mice with limb mesenchyme-specific Tfam deletions exhibited spontaneous limb bone fractures, resulting in severe limb deformities. Bone fragility was caused by poor apatite orientation owing to impaired osteoblast differentiation and maturation.






Similar content being viewed by others
References
Jeng JY, Yeh TS, Lee JW, Lin SH, Fong TH, Hsieh RH (2008) Maintenance of mitochondrial DNA copy number and expression are essential for preservation of mitochondrial function and cell growth. J Cell Biochem 103:347–357. https://doi.org/10.1002/jcb.21625
Yi CH, Pan H, Seebacher J, Jang IH, Hyberts SG et al (2011) Metabolic regulation of protein N-alpha-acetylation by Bcl-xL promotes cell survival. Cell 146:607–620. https://doi.org/10.1016/j.cell.2011.06.050
Feldenberg LR, Thevananther S, del Rio M, de Leon M, Devarajan P (1999) Partial ATP depletion induces Fas- and caspase-mediated apoptosis in MDCK cells. Am J Physiol 276:F837–F846. https://doi.org/10.1152/ajprenal.1999.276.6.F837
Sörensen L, Ekstrand M, Silva JP, Lindqvist E, Xu B, Rustin P, Olson L, Larsson NG (2001) Late-onset corticohippocampal neurodepletion attributable to catastrophic failure of oxidative phosphorylation in MILON mice. J Neurosci 21:8082–8090. https://doi.org/10.1523/jneurosci.21-20-08082.2001
Larsson NG, Clayton DA (1995) Molecular genetic aspects of human mitochondrial disorders. Annu Rev Genet 29:151–178. https://doi.org/10.1146/annurev.ge.29.120195.001055
Gaspari M, Larsson NG, Gustafsson CM (2004) The transcription machinery in mammalian mitochondria. Biochim Biophys Acta 1659:148–152. https://doi.org/10.1016/j.bbabio.2004.10.003
Parisi MA, Clayton DA (1991) Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science 252:965–969. https://doi.org/10.1126/science.2035027
Dairaghi DJ, Shadel GS, Clayton DA (1995) Addition of a 29 residue carboxyl-terminal tail converts a simple HMG box-containing protein into a transcriptional activator. J Mol Biol 249:11–28. https://doi.org/10.1006/jmbi.1995.9889
Ekstrand MI, Falkenberg M, Rantanen A, Park CB, Gaspari M, Hultenby K, Rustin P, Gustafsson CM, Larsson NG (2004) Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum Mol Genet 13:935–944. https://doi.org/10.1093/hmg/ddh109
Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 18:231–236. https://doi.org/10.1038/ng0398-231
Eriksen EF (2010) Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord 11:219–227. https://doi.org/10.1007/s11154-010-9153-1
Dirckx N, Moorer MC, Clemens TL, Riddle RC (2019) The role of osteoblasts in energy homeostasis. Nat Rev Endocrinol 15:651–665. https://doi.org/10.1038/s41574-019-0246-y
Dudley HR, Spiro D (1961) The fine structure of bone cells. J Biophys Biochem Cytol 11:627–649. https://doi.org/10.1083/jcb.11.3.627
Komarova SV, Ataullakhanov FI, Globus RK (2000) Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am J Physiol Cell Physiol 279:C1220–C1229. https://doi.org/10.1152/ajpcell.2000.279.4.C1220
Landis WJ (1995) The strength of a calcified tissue depends in part on the molecular structure and organization of its constituent mineral crystals in their organic matrix. Bone 16:533–544. https://doi.org/10.1016/8756-3282(95)00076-p
Nakano T, Kaibara K, Tabata Y, Nagata N, Enomoto S, Marukawa E, Umakoshi Y (2002) Unique alignment and texture of biological apatite crystallites in typical calcified tissues analyzed by microbeam x-ray diffractometer system. Bone 31:479–487. https://doi.org/10.1016/S8756-3282(02)00850-5
Ishimoto T, Nakano T, Umakoshi Y, Yamamoto M, Tabata Y (2013) Degree of biological apatite c-axis orientation rather than bone mineral density controls mechanical function in bone regenerated using recombinant bone morphogenetic protein-2. J Bone Miner Res 28:1170–1179. https://doi.org/10.1002/jbmr.1825
Ishimoto T, Sato B, Lee JW, Nakano T (2017) Co-deteriorations of anisotropic extracellular matrix arrangement and intrinsic mechanical property in c-src deficient osteopetrotic mouse femur. Bone 103:216–223. https://doi.org/10.1016/j.bone.2017.06.023
Sekita A, Matsugaki A, Ishimoto T, Nakano T (2017) Synchronous disruption of anisotropic arrangement of the osteocyte network and collagen/apatite in melanoma bone metastasis. J Struct Biol 197:260–270. https://doi.org/10.1016/j.jsb.2016.12.003
Ozasa R, Matsugaki A, Ishimoto T, Kamura S, Yoshida H, Magi M, Matsumoto Y, Sakuraba K, Fujimura K, Miyahara H, Nakano T (2022) Bone fragility via degradation of bone quality featured by collagen/apatite micro-arrangement in human rheumatic arthritis. Bone 155:116261. https://doi.org/10.1016/j.bone.2021.116261
Dobson PF, Dennis EP, Hipps D, Reeve A, Laude A, Bradshaw C, Stamp C, Smith A, Deehan DJ, Turnbull DM, Greaves LC (2020) Mitochondrial dysfunction impairs osteogenesis, increases osteoclast activity, and accelerates age related bone loss. Sci Rep 10:11643. https://doi.org/10.1038/s41598-020-68566-2
Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ (2002) Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33:77–80. https://doi.org/10.1002/gene.10092
Kawashima K, Ogawa H, Komura S, Ishihara T, Yamaguchi Y, Akiyama H, Matsumoto K (2020) Heparan sulfate deficiency leads to hypertrophic chondrocytes by increasing bone morphogenetic protein signaling. Osteoarthr Cartil 28:1459–1470. https://doi.org/10.1016/j.joca.2020.08.003
Nakanishi R, Akiyama H, Kimura H, Otsuki B, Shimizu M, Tsuboyama T, Nakamura T (2008) Osteoblast-targeted expression of Sfrp4 in mice results in low bone mass (in eng). J Bone Miner Res 23:271–277. https://doi.org/10.1359/jbmr.071007
Lee K, Deeds JD, Segre GV (1995) Expression of parathyroid hormone-related peptide and its receptor messenger ribonucleic acids during fetal development of rats. Endocrinology 136:453–463. https://doi.org/10.1210/endo.136.2.7835276
Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B (2002) The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev 16:2813–2828. https://doi.org/10.1101/gad.1017802
Bakker AD, Klein-Nulend J (2012) Osteoblast isolation from murine calvaria and long bones. Methods Mol Biol 816:19–29. https://doi.org/10.1007/978-1-61779-415-5_2
Sugahara S, Kume S, Chin-Kanasaki M, Tomita I, Yasuda-Yamahara M, Yamahara K, Takeda N, Osawa N, Yanagita M, Araki SI, Maegawa H (2019) Protein O-GlcNAcylation is essential for the maintenance of renal energy homeostasis and function via lipolysis during fasting and diabetes. J Am Soc Nephrol 30:962–978. https://doi.org/10.1681/ASN.2018090950
Hasegawa T, Yamamoto T, Sakai S, Miyamoto Y, Hongo H, Qiu Z, Abe M, Takeda S, Oda K, de Freitas PHL, Li M, Endo K, Amizuka N (2019) Histological effects of the combined administration of eldecalcitol and a parathyroid hormone in the metaphyseal trabeculae of ovariectomized rats. J Histochem Cytochem 67:169–184. https://doi.org/10.1369/0022155418806865
Hasegawa T, Endo T, Tsuchiya E, Kudo A, Shen Z, Moritani Y, Abe M, Yamamoto T, Hongo H, Tsuboi K, Yoshida T, Nagai T, Khadiza N, Yokoyama A, Luiz de Freitas PH, Li M, Amizuka N (2017) Biological application of focus ion beam-scanning electron microscopy (FIB-SEM) to the imaging of cartilaginous fibrils and osteoblastic cytoplasmic processes. J Oral Biosci 59:55–62. https://doi.org/10.1016/j.job.2016.11.004
Yamashita M, Inoue K, Saeki N, Ideta-Otsuka M, Yanagihara Y, Sawada Y, Sakakibara I, Lee J, Ichikawa K, Kamei Y, Iimura T, Igarashi K, Takada Y, Imai Y (2018) Uhrf1 is indispensable for normal limb growth by regulating chondrocyte differentiation through specific gene expression. Development. https://doi.org/10.1242/dev.157412
Iio H, Kikugawa T, Sawada Y, Sakai H, Yoshida S, Yanagihara Y, Ikedo A, Saeki N, Fukada SI, Saika T, Imai Y (2021) DNA maintenance methylation enzyme Dnmt1 in satellite cells is essential for muscle regeneration. Biochem Biophys Res Commun 534:79–85. https://doi.org/10.1016/j.bbrc.2020.11.116
Sakakibara I, Yanagihara Y, Himori K, Yamada T, Sakai H, Sawada Y, Takahashi H, Saeki N, Hirakawa H, Yokoyama A, Fukada SI, Sawasaki T, Imai Y (2021) Myofiber androgen receptor increases muscle strength mediated by a skeletal muscle splicing variant of Mylk4. iScience 24:102303. https://doi.org/10.1016/j.isci.2021.102303
da Huang W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. https://doi.org/10.1038/nprot.2008.211
Umeno A, Kotani H, Iwasaka M, Ueno S (2001) Quantification of adherent cell orientation and morphology under strong magnetic fields. IEEE Trans Magn 37:2909–2911
Ishimoto T, Nakano T, Yamamoto M, Tabata Y (2011) Biomechanical evaluation of regenerating long bone by nanoindentation. J Mater Sci Mater Med 22:969–976. https://doi.org/10.1007/s10856-011-4266-y
Oliver WC, Pharr GM (1992) An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res 7:1564–1583. https://doi.org/10.1557/JMR.1992.1564
Yao Q, Khan MP, Merceron C, LaGory EL, Tata Z, Mangiavini L, Hu J, Vemulapalli K, Chandel NS, Giaccia AJ, Schipani E (2019) Suppressing mitochondrial respiration is critical for hypoxia tolerance in the fetal growth plate. Dev Cell 49:748–63.e7. https://doi.org/10.1016/j.devcel.2019.04.029
Hsu YC, Wu YT, Yu TH, Wei YH (2016) Mitochondria in mesenchymal stem cell biology and cell therapy: from cellular differentiation to mitochondrial transfer. Semin Cell Dev Biol 52:119–131. https://doi.org/10.1016/j.semcdb.2016.02.011
Zheng CX, Sui BD, Qiu XY, Hu CH, Jin Y (2020) Mitochondrial regulation of stem cells in bone homeostasis. Trends Mol Med 26:89–104. https://doi.org/10.1016/j.molmed.2019.04.008
Shum LC, White NS, Mills BN, Bentley KL, Eliseev RA (2016) Energy metabolism in mesenchymal stem cells during osteogenic differentiation. Stem Cells Dev 25:114–122. https://doi.org/10.1089/scd.2015.0193
Yeh PS, Chen JT, Cherng YG, Yang ST, Tai YT, Chen RM (2020) Methylpiperidinopyrazole attenuates estrogen-induced mitochondrial energy production and subsequent osteoblast maturation via an estrogen receptor alpha-dependent mechanism. Molecules. https://doi.org/10.3390/molecules25122876
Burr DB (2019) Changes in bone matrix properties with aging. Bone 120:85–93. https://doi.org/10.1016/j.bone.2018.10.010
Nakano Y, Addison WN, Kaartinen MT (2007) ATP-mediated mineralization of MC3T3-E1 osteoblast cultures. Bone 41:549–561. https://doi.org/10.1016/j.bone.2007.06.011
Sroga GE, Vashishth D (2012) Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr Osteoporos Rep 10:141–150. https://doi.org/10.1007/s11914-012-0103-6
Moriishi T, Ozasa R, Ishimoto T, Nakano T, Hasegawa T, Miyazaki T, Liu W, Fukuyama R, Wang Y, Komori H, Qin X, Amizuka N, Komori T (2020) Osteocalcin is necessary for the alignment of apatite crystallites, but not glucose metabolism, testosterone synthesis, or muscle mass. PLoS Genet 16:e1008586. https://doi.org/10.1371/journal.pgen.1008586
Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH (2008) Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 26:960–968. https://doi.org/10.1634/stemcells.2007-0509
Rimessi A, Giorgi C, Pinton P, Rizzuto R (2008) The versatility of mitochondrial calcium signals: from stimulation of cell metabolism to induction of cell death. Biochim Biophys Acta 1777:808–816. https://doi.org/10.1016/j.bbabio.2008.05.449
Marchi S, Patergnani S, Missiroli S, Morciano G, Rimessi A, Wieckowski MR, Giorgi C, Pinton P (2018) Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 69:62–72. https://doi.org/10.1016/j.ceca.2017.05.003
Boonrungsiman S, Gentleman E, Carzaniga R, Evans ND, McComb DW, Porter AE, Stevens MM (2012) The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc Natl Acad Sci U S A 109:14170–14175. https://doi.org/10.1073/pnas.1208916109
Iwayama T, Okada T, Ueda T, Tomita K, Matsumoto S, Takedachi M, Wakisaka S, Noda T, Ogura T, Okano T, Fratzl P, Ogura T, Murakami S (2019) Osteoblastic lysosome plays a central role in mineralization. Science Adv 5:eaax0672. https://doi.org/10.1126/sciadv.aax0672
Primaditya V, Coryah FAN, Ariati LIP, Zakiah DWKK, Yuningsih PD, Khotimah H, Ali MM, Riawan W (2020) Effect of Centella asiatica to the glucose transporter 4 and osteocalcin on the rotenonee-induced zebrafish larvae (Danio rerio) stunting model. AIP Conf Proc 2231:040070. https://doi.org/10.1063/5.0002607
Acknowledgements
We thank L. Nils-Göran, T. Miyazaki and Y. Nakamichi for gifting the mice. We thank M. Hirosawa, K. Kondo, and M. Miwa for technical assistance. Our research was supported by the Japan Society for the Promotion of Science KAKENHI (JP18H02922).
Author information
Authors and Affiliations
Contributions
HY, SK and HA proposed the research project, designed and performed experiments, and wrote the manuscript. NK, AG, TH, TI, RO and YI performed experiments. NA and TN provided technical guidance.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
The experimental design and study protocols were approved by the Animal Experiment Committee of Gifu University and were performed in compliance with the Animal Research: Reporting of in Vivo Experimental (ARRIVE) guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
774_2022_1354_MOESM1_ESM.pdf
Supplementary file1 Supplemental Figure 1 (a) PCR analysis of genomic DNA isolated from the humerus of Tfam-cKO and Tfamf/f (control) mice at postnatal 6 days. (b) Expression of Tfam in the humerus at postnatal 6 days by qPCR (n=6, 3 biological replicates, 2 technical replicates) Supplemental Figure 2 (a) Immunofluorescence staining of Ki67 in the humerus at postnatal 6 days. The bars denote 100 μm. (b) TUNEL assay in hypertrophic zone (the upper frame) and in trabecular bone (the lower frame) to detect apoptosis in the humerus at postnatal 6 days. The bars denote 100 μm. (c) The number of TUNEL-positive cells in hypertrophic zone and in trabecular bone of humerus. The mean values ± SD and each value plot of the number of TUNEL-positive cells are shown (4 biological replicates) Supplemental Figure 3 Proteomic analysis using protein samples extracted from the cortex of the humerus in Tfam-cKO and control mice at postnatal 6 days (n=1, one biological sample). Col1a1: collagen type I alpha 1; Col1a2: collagen type I alpha 2 Supplemental Figure 4 Expression of SOD genes in the humerus at postnatal 6 days by qPCR. The mean values ± SD of the expression ratio to β-actin are shown (3 biological replicates and 3 technical replicates) (PDF 323 KB)
About this article
Cite this article
Yoshioka, H., Komura, S., Kuramitsu, N. et al. Deletion of Tfam in Prx1-Cre expressing limb mesenchyme results in spontaneous bone fractures. J Bone Miner Metab 40, 839–852 (2022). https://doi.org/10.1007/s00774-022-01354-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-022-01354-2