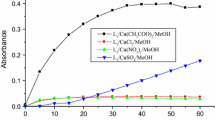
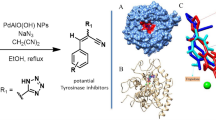
Abstract
Four new imidazole-based ligands, 4-((1H-imidazol-4-yl)methyl)-2-phenyl-4,5-dihydrooxyzole (L OL 1), 4-((1H-imidazol-4-yl)methyl)-2-(tert-butyl)-4,5-dihydrooxyzole (L OL 2), 4-((1H-imidazol-4-yl)methyl)-2-methyl-4,5-dihydrooxyzole (L OL 3), and N-(2,2-dimethylpropylidene)-2-(1-trityl-1H-imidazol-4-yl-)ethyl amine (L imz 1), have been synthesized. The corresponding copper(I) complexes [Cu(I)(L OL 1)(CH3CN)]PF6 (CuL OL 1), [Cu(I)(L OL 2)(CH3CN)]PF6 (CuL OL 2), [Cu(I)(L OL 3)(CH3CN)]PF6 (CuL OL 3), [Cu(I)(L imz 1)(CH3CN)2]PF6 (CuL imz 1) as well as the Cu(I) complex derived from the known ligand bis(1-methylimidazol-2-yl)methane (BIMZ), [Cu(I)(BIMZ)(CH3CN)]PF6 (CuBIMZ), are screened as catalysts for the oxidation of 3,5-di-tert-butylcatechol (3,5-DTBC-H2) to 3,5-di-tert-butylquinone (3,5-DTBQ). The primary reaction product of these oxidations is 3,5-di-tert-butylsemiquinone (3,5-DTBSQ) which slowly converts to 3,5-DTBQ. Saturation kinetic studies reveal a trend of catalytic activity in the order CuL OL 3 ≈ CuL OL 1 > CuBIMZ > CuL OL 2 > CuL imz 1. Additionally, the catalytic activity of the copper(I) complexes towards the oxygenation of monophenols is investigated. As substrates 2,4-di-tert-butylphenol (2,4-DTBP-H), 3-tert-butylphenol (3-TBP-H), 4-methoxyphenol (4-MeOP-H), N-acetyl-l-tyrosine ethyl ester monohydrate (NATEE) and 8-hydroxyquinoline are employed. The oxygenation products are identified and characterized with the help of UV/Vis and NMR spectroscopy, mass spectrometry, and fluorescence measurements. Whereas the copper complexes with ligands containing combinations of imidazole and imine functions or two imidazole units (CuL imz 1 and CuBIMZ) are found to exhibit catalytic tyrosinase activity, the systems with ligands containing oxazoline just mediate a stoichiometric conversion. Correlations between the structures of the complexes and their reactivities are discussed.



















Similar content being viewed by others
References
Solomon EI, Sundaram UM, Machonkin TE (1996) Chem Rev 96:2563–2605
Rolff M, Schottenheim J, Decker H, Tuczek F (2011) Chem Soc Rev 40:4077–4098
Decker H, Schweikardt T, Tuczek F (2006) Angew Chem Int Ed 45:4546–4550
Sánchez-Ferrer Á, Rodríguez-López JN, García-Cánovas F, García-Carmona F (1995) Biochim Biophys Acta 1247:1–11
Wu B (2014) Curr Top Med Chem 14:1425–1449
Simon JD, Peles D, Wakamatsu K, Ito S (2009) Pigment Cell Melanoma Res 22:563–579
Loizzo MR, Tundis R, Menichini F (2012) Compr Rev Food Sci Food Saf 11:378–398
van Holde KE, Miller KI, Decker H (2001) J Biol Chem 276:15563–15566
Solem E, Tuczek F, Decker H (2016) Angew Chem 128:2934–2938
Réglier M, Jorand C, Wagell B (1990) J Chem Soc Chem Commun 24:1752–1755
Casella L, Gullotti M, Bartosek M, Pallanza G, Laurenti E (1991) J Chem Soc Chem Commun 18:1235–1237
Battaini G, De Carolis M, Monzani E, Tuczek F, Casella L (2003) J Chem Soc Chem Commun 6:726–727
Battaini G, Monzani E, Casella L, Lonardi E, Tepper AWJW, Canters GW, Bubacco L (2002) J Biol Chem 277:44606–44612
Palavicini P, Granata A, Monzani E, Casella L (2005) J Am Chem Soc 127:18031–18036
Spada A, Palavicini S, Monzani E, Bubacco L, Casella L (2009) Dalton Trans 2009:6468–6471
Garcia-Bosch I, Company A, Frisch JR, Torrent-Sucarrat M, Cardellach M, Gamba I, Gìell M, Casella L, Que L Jr, Ribas X, Luis JM, Costas M (2010) Angew Chem Int Ed 49:2406–2409
Mirica LM, Vance M, Rudd DJ, Hedman B, Hodgson KO, Solomon EI, Stack TDP (2005) Science 308:1890–1892
Op’t Holt BT, Vance MA, Mirica LM, Heppner DE, Stack TDP, Solomon EI (2009) J Am Chem Soc 131:6421–6438
Rolff M, Schottenheim J, Peters G, Tuczek F (2010) Angew Chem Int Ed 49:6438–6442
Hoffmann A, Citek C, Binder S. Goos A, Rübhausen M, Troeppner O, Ivanovic-Burmazovic I, Wasinger EC, Stack TDP, Herres-Pawlis S (2013) Angew Chem Int Ed 52:5398–5401
Esguerra KVN, Fall Y, Lumb JP (2014) Angew Chem Int Ed 53:5877–5881
Askari MS, Rodriguez-Solano LA, Proppe A, McAllister B, Lumb JP, Otterwaelder X (2015) Dalton Trans 44:12094–12097
Xu B, Lumb JP, Arndtsen BA (2015) Angew Chem Int Ed 54:4208–4211
Esguerra KVN, Fall Y, Petijean L, Lumb JP (2014) J Am Chem Soc 136:7662–7668
Askari MS, Esguerra KVN, Lumb JP, Ottenwaelder X (2015) Inorg Chem 54:8665–8672
Huang Z, Kwon O, Esguerra KVN, Lumb JP (2015) Tetrahedron 71:5871–5885
Hamann JN, Schneider R, Tuczek F (2015) J Coord Chem 68:3259–3271
Schottenheim J, Gernert C, Herzigkeit B, Krahmer J, Tuczek F (2015) Eur J Inorg Chem 2015:3501–3511
Hamann JN, Rolff M, Tuczek F (2015) Dalton Trans 44:3251–3258
Hamann JN, Tuczek F (2014) Chem Commun 50:2298–2300
Schottenheim J, Fateeva N, Thimm W, Krahmer J, Tuczek F (2013) Z Allg Anorg Chem 8:1491–1497
Rolff M, Hamann JN, Tuczek F (2011) Angew Chem 123:7057–7061
Rolff M, Schottenheim J, Tuczek F (2010) J Coord Chem 63:2382–2399
Rolff M, Tuczek F (2008) Angew Chem 120:2378–2381
Braussaud N, Rüther T, Cavell KJ, Skelton BW, White AH (2001) Synthesis 4:626–632
Kovalainen JT, Christiaans JAM, Kotisaari S, Laitinen JT, Männistö PT, Tuomisto L, Gynther J (1999) J Med Chem 42:1193–1202
Garibay PW (2011) US 2011/0166321 A1
Kupfer R, Nagel M, Wuerthwein EU, Allmann R (1985) Chem Ber 118:3089–3104
Sheldrick GM (2008) Acta Crystallogr Sect A Found Crystallogr 64:112–122
Sheldrick GM (2015) Acta Crystallogr C 71:3–8
Monzani E, Quinti L, Perotti A, Casella L, Gullotti M, Randaccio L, Geremia S, Nardin G, Faleschini P, Tabbi G (1998) Inorg Chem 37:553–562
Mukherjee J, Mukherjee R (2002) Inorg Chim Acta 337:429–438
Nevesa A, Rossi LM, Bortoluzzi AJ, Szpoganicz B, Wiezbicki C, Schwingel E (2002) Inorg Chem 41:1788–1794
Sénèque O, Campion M, Douziech B, Giorgi M, Rivière E, Journaux Y, Le Mest Y, Reinaud O (2002) Eur J Inorg Chem 8:2007–2014
Rall J, Wanner M, Albrecht M, Hornung FM, Kaim W (1999) Chem Eur J 5:2802–2809
Horner L, Geyer E (1965) Chem Ber 98:2016–2045
Harmalker S, Jones SE, Sawyer DT (1983) Inorg Chem 22:2790–2794
Stallings MD, Morrison MM, Sawyer DT (1981) Inorg Chem 20:2655–2660
Gentschev P, Müller N, Krebs B (2000) Inorg Chim Acta 300:422–452
Zippel F, Ahlers F, Werner R, Haase W, Nolting HF, Krebs B (1996) Inorg Chem 35:3409–3419
Wegner R, Gottschaldt M, Görls H, Jäger EG, Klemm D (2000) Angew Chem 112:608–612
Kao CH, Wie HH, Liu YH, Lee GH, Wang Y, Lee CJ (2001) J Inorg Biochem 84:171–178
Wegner R, Gottschaldt M, Görls H, Jäger EG, Klemm D (2001) Chem Eur J 7:2143–2157
Ackermann J, Meyer F, Kaifer E, Pritzkow H (2002) Chem Eur J 8:247–258
Manzur J, Garcia AM, Rivas V, Atria AM, Valenzuela J, Spodine E (1997) Polyhedron 16:2299–2301
Jovanovic SV, Kónya K, Scaiano JC (1995) Can J Chem 73:1803–1810
Bulkowski JE (1985) US patent 4545937
Ramadan AEMM, Youssef S, Eissa H (2014) Int J Adv Res 2:116–130
Clayden J, Greeves N, Warren S (2012) Organic chemistry. Oxford University Press, Oxford
Nilges MJ, Swartz HM, Riley PA (1984) J Biol Chem 259:2446–2451
Taylor SW, Molinski TF, Rzepecki LM, Waite JH (1991) J Nat Prod 54:918–922
Badger GM, Walker IS (1956) J Chem Soc, pp 122–126
Zhu JH, Olmstead JA, Gray DG (1995) J Wood Chem Technol 15:43–64
Matoba Y, Kumagai T, Yamamoto A, Yoshitsu H, Sugiyama M (2006) J Biol Chem 281:8981–8990
Wilfer C, Liebhäuser P, Hoffmann A, Erdmann H, Grossmann O, Runtsch L, Paffenholz E, Schepper R, Dick R, Bauer M, Dürr M, Ivanovic-Burmazovic I, Herres-Pawlis S (2015) Chem Eur J 21:17639–17649
Palenik GJ (1964) Acta Cryst 17:687–695
Walli A, Dechert S, Bauer M, Demeshko S, Meyer F (2014) Eur J Inorg Chem 2014:4660–4676
Li J, Widlicka DW, Fichter K, Reed DP, Weisman GR, Wong EH, DiPasquale A, Heroux KJ, Golen JA, Reinhold AL (2010) Inorg Chim Acta 364:185–194
Santagostini L, Gullotti M, Monzani E, Casella L, Dillinger R, Tuczek F (2000) Chem Eur J 6:519–522
Acknowledgments
We express our gratitude to Deutsche Forschungsgemeinschaft (DFG), CAU Kiel and COST CM 1003 for support of this research. Thanks to Miriam Schehr for the introduction to operate with the Isolera One fabricated by Biotage, Marcel Dommaschk for the help measuring the fluorescence spectra and Michael Wendt for performing the XRPD measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there are no conflicts of interests.
Additional information
Dedicated to Prof. Dr. Edward I. Solomon in honor of the ACS Alfred Bader Award.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wendt, F., Näther, C. & Tuczek, F. Tyrosinase and catechol oxidase activity of copper(I) complexes supported by imidazole-based ligands: structure–reactivity correlations. J Biol Inorg Chem 21, 777–792 (2016). https://doi.org/10.1007/s00775-016-1370-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-016-1370-y