Abstract
Objectives
The purpose of the present systematic review and meta-analysis is to determine the efficacy of antibiotic prophylaxis and specific antibiotic regimens in dental implant placement for prevention of post-operative infection (POI) in overall healthy patients.
Materials and methods
Electronic database and manual searches were independently conducted to identify randomized controlled trials (RCTs). Publications were selected on basis of eligibility criteria and then assessed for risk-of-bias using the Cochrane Handbook. The primary outcome was POI (total, early, and late). Wound dehiscence, pain, and adverse events were studied as secondary outcomes. Random-effects meta-analysis was conducted for risk ratios of dichotomous data. This systematic review was conducted in accordance with Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.
Results
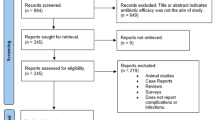
With duplicates removed, 1022 abstracts were screened and 22 full-text articles assessed; 10 RCTs of 1934 total patients were included. Meta-analysis did not detect statistically significant differences in total (P = 0.82), early (1–2 week post-op) (P = 0.57), or late (3–4 months post-op) (P = 0.66) POIs, wound dehiscence (P = 0.31), and adverse events (P = 0.21), between antibiotic and no-antibiotic groups. Confounding variables identified.
Conclusion
The results of this systematic review suggest that antibiotic prophylaxis may not be indicated for prevention of POIs following dental implant placement in overall healthy patients. These findings and in light of antibiotic-associated risks for individual and public health demand revaluation of routine prescription of antibiotic prophylaxis in dental implant placement procedures.
Clinical relevance
It is up to the clinicians to evaluate the benefits (or lack thereof) of antibiotic prophylaxis for each patient given medical history and surgical complexity, until new evidence becomes available.




Similar content being viewed by others
References
Peterson LJ (1990) Antibiotic prophylaxis against wound infections in oral and maxillofacial surgery. J Oral Maxillofac Surg 48(6):617–620
Burke JF (1961) The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery 50:161–168
Woods RK, Dellinger EP (1998) Current guidelines for antibiotic prophylaxis of surgical wounds. Am Fam Physician 57(11):2731–2740
Olson M, O'Connor M, Schwartz ML (1984) Surgical wound infections. A 5-year prospective study of 20,193 wounds at the Minneapolis VA Medical Center. Ann Surg 199(3):253–259
Page CP, Bohnen JM, Fletcher JR, McManus AT, Solomkin JS, Wittmann DH (1993) Antimicrobial prophylaxis for surgical wounds. Guidelines for clinical care. Arch Surg (Chicago, Ill : 1960) 128(1):79–88
Esposito M, Grusovin MG, Worthington HV (2013) Interventions for replacing missing teeth: antibiotics at dental implant placement to prevent complications. The Cochrane database of systematic reviews (7):Cd004152. doi:https://doi.org/10.1002/14651858.CD004152.pub4
Ata-Ali J, Ata-Ali F, Ata-Ali F (2014) Do antibiotics decrease implant failure and postoperative infections? A systematic review and meta-analysis. Int J Oral Maxillofac Surg 43(1):68–74. https://doi.org/10.1016/j.ijom.2013.05.019
Chrcanovic BR, Albrektsson T, Wennerberg A (2014) Prophylactic antibiotic regimen and dental implant failure: a meta-analysis. J Oral Rehabil 41(12):941–956. https://doi.org/10.1111/joor.12211
Sharaf B, Dodson TB (2011) Does the use of prophylactic antibiotics decrease implant failure? Oral Maxillofac Surg Clin North Am 23(4):547–550, vi. https://doi.org/10.1016/j.coms.2011.07.008
Bryce G, Bomfim DI, Bassi GS (2014) Pre- and post-operative management of dental implant placement. Part 2: management of early-presenting complications. Br Dent J 217(4):171–176. https://doi.org/10.1038/sj.bdj.2014.702
Surapaneni H, Yalamanchili PS, Basha MH, Potluri S, Elisetti N, Kiran Kumar MV (2016) Antibiotics in dental implants: a review of literature. J Pharm Bioallied Sci 8(Suppl 1):S28–s31. https://doi.org/10.4103/0975-7406.191961
Lund B, Hultin M, Tranaeus S, Naimi-Akbar A, Klinge B (2015) Complex systematic review—perioperative antibiotics in conjunction with dental implant placement. Clin Oral Implants Res 26 Suppl 11:1–14. https://doi.org/10.1111/clr.12637
Park J, Tennant M, Walsh L, Kruger E (2018) Is there a consensus on antibiotic usage for dental implant placement in healthy patients? Aust Dent J 63(1):25–33
Schwartz AB, Larson EL (2007) Antibiotic prophylaxis and postoperative complications after tooth extraction and implant placement: a review of the literature. J Dent 35(12):881–888. https://doi.org/10.1016/j.jdent.2007.08.003
Termine N, Panzarella V, Ciavarella D, Lo Muzio L, D'Angelo M, Sardella A, Compilato D, Campisi G (2009) Antibiotic prophylaxis in dentistry and oral surgery: use and misuse. Int Dent J 59(5):263–270
Ahmad N, Saad N (2012) Effects of antibiotics on dental implants: a review. J Clin Med Res 4(1):1–6. https://doi.org/10.4021/jocmr658w
Resnik RR, Misch C (2008) Prophylactic antibiotic regimens in oral implantology: rationale and protocol. Implant Dent 17(2):142–150. https://doi.org/10.1097/ID.0b013e3181752b09
Laskin DM, Dent CD, Morris HF, Ochi S, Olson JW (2000) The influence of preoperative antibiotics on success of endosseous implants at 36 months. Annals Periodontology 5(1):166–174. https://doi.org/10.1902/annals.2000.5.1.166
Quirynen M, De Soete M, van Steenberghe D (2002) Infectious risks for oral implants: a review of the literature. Clin Oral Implants Res 13(1):1–19
Shehab N, Patel PR, Srinivasan A, Budnitz DS (2008) Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis 47(6):735–743
Ziment I, Barrett VD, Beall GN, Glassock RJ, Locks MO, Lubran M, Nelson JR, Reisner RM, Tanaka KR (1972) Complications of antibiotic therapy. Paper presented at the Teaching Conference in Infectious Diseases, University of California, Los Angeles. Harbor General Hospital, Torrance, November 1972
Viola M, Quaratino D, Gaeta F, Valluzzi RL, Caruso C, Rumi G, Romano A (2005) Allergic reactions to antibiotics, mainly betalactams: facts and controversies. Eur Ann Allergy Clin Immunol 37(6):223–229
New HC (1979) Prophylaxis—has it at last come of age? J Antimicrob Chemother 5(4):331
Andersson DI, Hughes D (2011) Persistence of antibiotic resistance in bacterial populations. FEMS Microbiol Rev 35(5):901–911
Sundqvist M (2014) Reversibility of antibiotic resistance. Ups J Med Sci 119(2):142–148. https://doi.org/10.3109/03009734.2014.903323
Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M (2014) A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis 14:13. https://doi.org/10.1186/1471-2334-14-13
Llewelyn MJ, Fitzpatrick JM, Darwin E, SarahTonkin C, Gorton C, Paul J, Peto TEA, Yardley L, Hopkins S, Walker AS (2017) The antibiotic course has had its day. BMJ (Clinical research ed) 358:j3418. https://doi.org/10.1136/bmj.j3418
Arason VA, Kristinsson KG, Sigurdsson JA, Stefansdottir G, Molstad S, Gudmundsson S (1996) Do antimicrobials increase the carriage rate of penicillin resistant pneumococci in children? Cross sectional prevalence study. BMJ (Clinical research ed) 313(7054):387–391
Lockhart PB, Blizzard J, Maslow AL, Brennan MT, Sasser H, Carew J (2013) Drug cost implications for antibiotic prophylaxis for dental procedures. Oral Surg Oral Med Oral Pathol Oral Radiol 115(3):345–353. https://doi.org/10.1016/j.oooo.2012.10.008
Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, Fish DN, Napolitano LM, Sawyer RG, Slain D, Steinberg JP, Weinstein RA (2013) Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health-Syst Pharm : AJHP 70(3):195–283. https://doi.org/10.2146/ajhp120568
Bratzler DW, Houck PM (2005) Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg 189(4):395–404. https://doi.org/10.1016/j.amjsurg.2005.01.015
WHO (2014) Antimicrobial resistance: global report on surveillance. http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1
Tan WC, Ong M, Han J, Mattheos N, Pjetursson BE, Tsai AY-M, Sanz I, Wong MCM, Lang NP, on Behalf of the ITIASG (2014) Effect of systemic antibiotics on clinical and patient-reported outcomes of implant therapy—a multicenter randomized controlled clinical trial. Clin Oral Implants Res 25(2):185–193. https://doi.org/10.1111/clr.12098
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed) 339:b2700. https://doi.org/10.1136/bmj.b2700
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Higgins JP (2011) Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. wwwcochrane-handbookorg
Alsaadi G, Quirynen M, Michiles K, Teughels W, Komarek A, van Steenberghe D (2008) Impact of local and systemic factors on the incidence of failures up to abutment connection with modified surface oral implants. J Clin Periodontol 35(1):51–57. https://doi.org/10.1111/j.1600-051X.2007.01165.x
Dent CD, Olson JW, Farish SE, Bellome J, Casino AJ, Morris HF, Ochi S (1997) The influence of preoperative antibiotics on success of endosseous implants up to and including stage II surgery: a study of 2,641 implants. J Oral Maxillofac Surg 55(12 Suppl 5):19–24
Lambert PM, Morris HF, Ochi S (2000) The influence of smoking on 3-year clinical success of osseointegrated dental implants. Ann Periodontol 5(1):79–89. https://doi.org/10.1902/annals.2000.5.1.79
Escalante MG, Eubank TD, Leblebicioglu B, Walters JD (2015) Comparison of azithromycin and amoxicillin before dental implant placement: an exploratory study of bioavailability and resolution of postoperative inflammation. J Periodontol 86(11):1190–1200. https://doi.org/10.1902/jop.2015.150024
Larsen P, McGlumphy E (1993) Antibiotic prophylaxis for placement of dental implants. J Oral Maxillofac Surg 51:194
Manz MC (2000) Factors associated with radiographic vertical bone loss around implants placed in a clinical study. Ann Periodontol 5(1):137–151. https://doi.org/10.1902/annals.2000.5.1.137
Morris HF, Ochi S, Plezia R, Gilbert H, Dent CD, Pikulski J, Lambert PM (2004) AICRG, part III: the influence of antibiotic use on the survival of a new implant design. J Oral Implantol 30(3):144–151. https://doi.org/10.1563/1548-1336(2004)30<144:apitio>2.0.co;2
Orenstein IH, Petrazzuolo V, Morris HF, Ochi S (2000) Variables affecting survival of single-tooth hydroxyapatite-coated implants in anterior maxillae at 3 years. Annals of periodontology 5(1):68–78. https://doi.org/10.1902/annals.2000.5.1.68
Binahmed A, Stoykewych A, Peterson L (2005) Single preoperative dose versus long-term prophylactic antibiotic regimens in dental implant surgery. Int J Oral Maxillofac Implants 20(1):115–117
Karaky AE, Sawair FA, Al-Karadsheh OA, Eimar HA, Algarugly SA, Baqain ZH (2011) Antibiotic prophylaxis and early dental implant failure: a quasi-random controlled clinical trial. Eur J Oral Implantol 4(1):31–38
Abu-Ta'a M, Quirynen M, Teughels W, van Steenberghe D (2008) Asepsis during periodontal surgery involving oral implants and the usefulness of peri-operative antibiotics: a prospective, randomized, controlled clinical trial. J Clin Periodontol 35 (1):58–63. doi:https://doi.org/10.1111/j.1600-051X.2007.01162.x
Anitua E, Aguirre JJ, Gorosabel A, Barrio P, Errazquin JM, Roman P, Pla R, Carrete J, de Petro J, Orive G (2009) A multicentre placebo-controlled randomised clinical trial of antibiotic prophylaxis for placement of single dental implants. Eur J Oral Implantol 2(4):283–292
Arduino PG, Tirone F, Schiorlin E, Esposito M (2015) Single preoperative dose of prophylactic amoxicillin versus a 2-day postoperative course in dental implant surgery: a two-centre randomised controlled trial. Eur J Oral Implantol 8(2):143–149
Caiazzo A, Casavecchia P, Barone A, Brugnami F (2011) A pilot study to determine the effectiveness of different amoxicillin regimens in implant surgery. J Oral Implantol 37(6):691–696. https://doi.org/10.1563/aaid-joi-d-09-00134.1
El-Kholey KE (2014) Efficacy of two antibiotic regimens in the reduction of early dental implant failure: a pilot study. International Journal of Oral & Maxillofacial Surgery 43(4):487–490. https://doi.org/10.1016/j.ijom.2013.09.013
Esposito M, Cannizzaro G, Bozzoli P, Consolo U, Felice P, Ferri V, Landriani S, Leone M, Magliano A, Pellitteri G, Todisco M, Torchio C (2008) Efficacy of prophylactic antibiotics for dental implants: a multicentre placebo-controlled randomised clinical trial. Eur J Oral Implantol 1(1):23–31
Esposito M, Cannizzaro G, Bozzoli P, Checchi L, Ferri V, Landriani S, Leone M, Todisco M, Torchio C, Testori T, Galli F, Felice P (2010) Effectiveness of prophylactic antibiotics at placement of dental implants: a pragmatic multicentre placebo-controlled randomised clinical trial. Eur J Oral Implantol 3(2):135–143
Nolan R, Kemmoona M, Polyzois I, Claffey N (2014) The influence of prophylactic antibiotic administration on post-operative morbidity in dental implant surgery. A prospective double blind randomized controlled clinical trial. Clin Oral Implants Res 25(2):252–259. https://doi.org/10.1111/clr.12124
Moslemi N, Shahnaz A, Bahador A, Torabi S, Jabbari S, Oskouei ZA (2016) Effect of postoperative amoxicillin on early bacterial colonization of peri-implant sulcus: a randomized controlled clinical trial. J Dentistry (Tehran, Iran) 13(5):309–317
Kashani H, Dahlin C, Alse'n B (2005) Influence of different prophylactic antibiotic regimens on implant survival rate: a retrospective clinical study. Clin Implant Dent Relat Res 7(1):32–35
Bruce J, Russell EM, Mollison J, Krukowski ZH (2001) The quality of measurement of surgical wound infection as the basis for monitoring: a systematic review. The Journal of hospital infection 49(2):99–108. https://doi.org/10.1053/jhin.2001.1045
Leaper D, Tanner J, Kiernan M (2013) Surveillance of surgical site infection: more accurate definitions and intensive recording needed. J Hosp Infect 83(2):83–86. https://doi.org/10.1016/j.jhin.2012.11.013
Esposito M, Grusovin MG, Loli V, Coulthard P, Worthington HV (2010) Does antibiotic prophylaxis at implant placement decrease early implant failures? A Cochrane systematic review. Eur J Oral Implantol 3(2):101–110
Quirynen M, Vogels R, Alsaadi G, Naert I, Jacobs R, Steenberghe D (2005) Predisposing conditions for retrograde peri-implantitis, and treatment suggestions. Prädisponierende Faktoren für die retrograde Peri-Implantitis und Vorschläge zur Behandlung. Clin Oral Implants Res 16(5):599–608. https://doi.org/10.1111/j.1600-0501.2005.01147.x
McAllister B, Masters D, M Meffert R (1992) Treatment of implants demonstrating periapical radiolucencies, vol 4
Scarano A, Di Domizio P, Petrone G, Iezzi G, Piattelli A (2000) Implant periapical lesion: a clinical and histologic case report. The Journal of oral implantology 26(2):109–113. https://doi.org/10.1563/1548-1336(2000)026<0109:iplaca>2.3.co;2
Sadig W, Almas K (2004) Risk factors and management of dehiscent wounds in implant dentistry. Implant Dent 13(2):140–147
Sandy-Hodgetts K, Carville K, Leslie GD (2015) Determining risk factors for surgical wound dehiscence: a literature review. Int Wound J 12(3):265–275. https://doi.org/10.1111/iwj.12088
Rizzo S, Zampetti P, Rodriguez YBR, Svanosio D, Lupi SM (2010) Retrospective analysis of 521 endosseous implants placed under antibiotic prophylaxis and review of literature. Minerva Stomatol 59(3):75–88
Chen Z, Chen D, Zhang S, Tang L, Li Q (2017) Antibiotic prophylaxis for preventing dental implant failures and postoperative infection: a systematic review of randomized controlled trials. Am J Dent 30(2):89–95
Petty W, Spanier S, Shuster JJ, Silverthorne C (1985) The influence of skeletal implants on incidence of infection. Experiments in a canine model. The Journal of bone and joint surgery American 67(8):1236–1244
Francetti L, Del Fabbro M, Basso M, Testori T, Taschieri S, Weinstein R (2004) Chlorhexidine spray versus mouthwash in the control of dental plaque after implant surgery. J Clin Periodontol 31(10):857–862. https://doi.org/10.1111/j.1600-051X.2004.00566.x
D'Ercole S, Tete S, Catamo G, Sammartino G, Femminella B, Tripodi D, Spoto G, Paolantonio M (2009) Microbiological and biochemical effectiveness of an antiseptic gel on the bacterial contamination of the inner space of dental implants: a 3-month human longitudinal study. Int J Immunopathol Pharmacol 22(4):1019–1026. https://doi.org/10.1177/039463200902200417
Lambert PM, Morris HF, Ochi S (1997) The influence of 0.12% chlorhexidine digluconate rinses on the incidence of infectious complications and implant success. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 55(12 Suppl 5):25–30
Young MP, Korachi M, Carter DH, Worthington HV, McCord JF, Drucker DB (2002) The effects of an immediately pre-surgical chlorhexidine oral rinse on the bacterial contaminants of bone debris collected during dental implant surgery. Clin Oral Implants Res 13(1):20–29
Bryce G, Bomfim DI, Bassi GS (2014) Pre- and post-operative management of dental implant placement. Part 1: management of post-operative pain. Br Dent J 217(3):123–127. https://doi.org/10.1038/sj.bdj.2014.650
Acknowledgments
We are grateful to Mr. Richard McGowan (NYU Health Sciences Library liaison to the NYU Dentistry) for his assistance with the electronic database search process.
Our appreciation extends to Dr. Analia Vietz-Keenan (NYU Dentistry Department of Oral and Maxillofacial Pathology, Radiology, & Medicine) and Dr. Malvin N. Janal (NYU Dentistry Department of Epidemiology and Health Promotion) for sharing with us their expert opinions on evidence based dentistry and statistical analyses of results.
We thank fellow researchers, Dr. Eduardo Anitua, Dr. Alfonso Caiazzo, Dr. Khalid E. El-Kholey, Dr. Marco Esposito, and Dr. Ioannis Polyzois (Nolan 2012 group), for kindly providing clarification with regard to study design and/or results.
Funding
This systematic review received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
I. Khouly and R. S. Braun contributed to study conception and design, acquisition of data, analysis and interpretation, and drafted the manuscript. L. Chambrone contributed to study analysis, interpretation of data, and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this paper declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. Ethical approval is not required.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1. Search protocol used in the systematic review
The following search terms, created by a medical-dental librarian, were used for database searches.
Primary concept
Keywords: endosseous implant OR endosseous implants OR dental implant OR dental implants OR dental implantation OR tooth implant OR tooth implants OR tooth implantation
Subject headings: PubMed and CINAHL: “dental implantation, endosseous”[MeSH Terms] OR “dental implantation”[MeSH Terms] OR “dental implants”[MeSH Terms]
EMBASE: tooth implant/ OR tooth implantation/(EMTREE)
DOSS: uses keywords only
Secondary concept
Keywords: antibiotic OR antibiotics OR (antibiotic prophylaxis) OR (prophylactic antibiotic) OR (prophylactic antibiotics) OR (antibiotic regimen) OR (pre-operative antibiotic) OR (post-operative antibiotic) OR (postoperative antibiotic) OR (perioperative antibiotics) OR (systemic antibiotic) OR (infection prevention)
Subject headings: PubMed and CINAHL: “antibiotic prophylaxis”[MeSH Terms] OR “anti-bacterial agents”[MeSH Terms]
EMBASE: antibiotics prophylaxis/ (EMTREE)
DOSS: uses keywords only
Detailed search queries were as follows:
PubMed: (((“antibiotic”[All Fields] OR “antibiotics”[All Fields] OR “antibiotic prophylaxis”[MeSH Terms] OR (“antibiotic”[All Fields] AND “prophylaxis”[All Fields]) OR “antibiotic prophylaxis”[All Fields] OR (prophylactic[All Fields] AND “antibiotic”[All Fields]) OR (prophylactic[All Fields] AND “antibiotics”[All Fields]) OR (“antibiotic”[All Fields] AND “regimen”[All Fields]) OR (pre-operative[All Fields] AND “antibiotic”[All Fields]) OR (pre-operative[All Fields] AND “antibiotics”[All Fields]) OR ((“postoperative period”[MeSH Terms] OR “postoperative”[All Fields] OR “postoperative period”[All Fields] OR “post operative”[All Fields]) AND “antibiotic”[All Fields]) OR ((“postoperative period”[MeSH Terms] OR “postoperative”[All Fields] OR “postoperative period”[All Fields] OR “post operative”[All Fields]) AND “antibiotics”[All Fields]) OR (perioperative[All Fields] AND “antibiotic”[All Fields]) OR (perioperative[All Fields] AND “antibiotics”[All Fields]) OR (systemic[All Fields] AND “antibiotic”[All Fields]) OR (systemic[All Fields] AND “antibiotics”[All Fields]) OR ((“infection”[MeSH Terms] OR “infection”[All Fields]) AND (“prevention and control”[Subheading] OR “prevention and control”[All Fields] OR “prevention”[All Fields]))))) AND ((“dental implants”[MeSH Terms] OR (“dental”[All Fields] AND “implants”[All Fields]) OR “dental implants”[All Fields] OR (“dental”[All Fields] AND “implant”[All Fields]) OR “dental implant”[All Fields] OR “dental implantation”[MeSH Terms] OR (“dental”[All Fields] AND “implantation”[All Fields]) OR “dental implantation”[All Fields] OR ((“tooth”[MeSH Terms] OR “tooth”[All Fields]) AND implant[All Fields]) OR ((“tooth”[MeSH Terms] OR “tooth”[All Fields]) AND implants[All Fields]) OR ((“tooth”[MeSH Terms] OR “tooth”[All Fields]) AND “implantation”[All Fields]) OR (endosseous[All Fields] AND implant[All Fields]) OR “endosseous implant”[All Fields] OR (“endosseous”[All Fields] AND “implants”[All Fields]) OR “endosseous implants”[All Fields] OR “dental implantation, endosseous”[MeSH Terms] OR “endosseous dental implantation”[All Fields]))
CINAHL: ((MH “Antibiotic Prophylaxis” OR MH “Antibiotics” OR Antibiotic OR antibiotics OR (antibiotic prophylaxis) OR (prophylactic antibiotic) OR (prophylactic antibiotics) OR (antibiotic regimen) OR (pre-operative antibiotic) OR (post-operative antibiotic) OR (postoperative antibiotic) OR (perioperative antibiotics) OR (systemic antibiotic) OR (infection prevention)) AND (endosseous implant OR endosseous implants OR dental implant OR dental implants OR dental implantation OR tooth implant OR tooth implants OR Tooth implantation OR MH “Dental Implants” OR MH “Dental Implantation”)
DOSS: (((Antibiotic OR antibiotics OR (antibiotic prophylaxis) OR (prophylactic antibiotic) OR (prophylactic antibiotics) OR (antibiotic regimen) OR (pre-operative antibiotic) OR (post-operative antibiotic) OR (postoperative antibiotic) OR (perioperative antibiotics) OR (systemic antibiotic) OR (infection prevention))) AND ((endosseous implant OR endosseous implants OR dental implant OR dental implants OR dental implantation OR tooth implant OR tooth implants OR Tooth implantation))
EMBASE: (exp antibiotic prophylaxis/ OR (Antibiotic OR antibiotics OR (antibiotic prophylaxis) OR (prophylactic antibiotic) OR (prophylactic antibiotics) OR (antibiotic regimen) OR (pre-operative antibiotic) OR (post-operative antibiotic) OR (postoperative antibiotic) OR (perioperative antibiotics) OR (systemic antibiotic) OR (infection prevention)).mp.)
AND (exp tooth implant/ OR exp. tooth implantation/ OR (endosseous implant or endosseous implants or dental implant or dental implants or dental implantation or tooth implant or tooth implants or Tooth implantation).mp.)
Appendix 2. Journals included in handsearch
The journals included in handsearch were as follows:
International Journal of Oral and Maxillofacial Implants, European Journal of Oral Implantology, Clinical Oral Implants Research, International Journal of Oral and Maxillofacial Surgery, British Journal of Oral and Maxillofacial Surgery, Journal of Oral and Maxillofacial Surgery, Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontology, International Journal of Oral and Maxillofacial Surgery Clinical Implant Dentistry and Related Research, Dental Clinics of North America, Journal of Prosthetic Dentistry, Journal of Clinical Periodontology, Journal of Oral Implantology, Journal of Craniofacial Surgery, Journal of Periodontology, Journal of Cranio-Maxillofacial Surgery.
Appendix 3. Extracted information on study characteristics
Extracted information on study characteristics included publication details (author(s), year of publication), trial study design/study design, source of funding, conflict of interest, study period, and location. Treatment group characteristics included the study group intervention (prophylactic antibiotic compound, dosage, and duration of treatment), comparative treatment (placebo or another prophylactic antibiotic with specification of regimen), number of patients and implants, mean age/range, number of females and males, and patient dental and medical history (periodontitis, smoking, diabetes). Treatment/surgical protocols specified additional protocol measures (administration of chlorohexidine, dexamethasone, etc.), implant placement stages (one or two stages), bone graft/graft type, implant platform, and healing/loading protocol. Study outcomes included a summary of outcomes assessed, follow-up times, and study conclusions as well as quantitative results for outcomes assessed: post-operative infection (total, early, and late infections), wound dehiscence, pain, and adverse events. All outcomes were reported in terms of patient number or as reported in the publications if patient number could not be obtained (e.g., pain was often recorded as a mean VAS score). Extracted data were categorized on the basis of antibiotic prophylaxis regimens: administration of only pre-operative antibiotics, pre- and post-operative antibiotics, only post-operative antibiotics, and no antibiotics and respective antibiotic dosage for each regimen.
Section/topic | No. | Checklist item | Reported on page no. |
|---|---|---|---|
TITLE | |||
Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
ABSTRACT | |||
Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 2 |
INTRODUCTION | |||
Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 3–4 |
Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 4 |
METHODS | |||
Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | 4 |
Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 4–5 |
Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 5 |
Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 6 |
Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 6 |
Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 6 |
Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 6 |
Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 6 |
Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 6–7 |
Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 6–7 |
Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 6 |
Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 6 |
RESULTS | |||
Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 7 |
Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 7 |
Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 9–10 |
Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 9–10 |
Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 8–9 |
Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 9–10 |
Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | 9 |
DISCUSSION | |||
Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 10 |
Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 12 |
Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 13 |
FUNDING | |||
Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 1 |
Differences in total post-operative infection at 1–2 weeks. a Pre-operative only antibiotic group. b Pre- and post-operative antibiotic group. c Pre- and post-operative/post-operative only antibiotic group. d Only antibiotic regimen versus placebo. e Pre-operative antibiotic only versus pre- and post-operative/post-operative only antibiotic
Rights and permissions
About this article
Cite this article
Khouly, I., Braun, R.S. & Chambrone, L. Antibiotic prophylaxis may not be indicated for prevention of dental implant infections in healthy patients. A systematic review and meta-analysis. Clin Oral Invest 23, 1525–1553 (2019). https://doi.org/10.1007/s00784-018-2762-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2762-x