Abstract
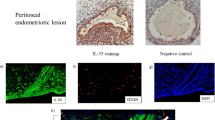
Endometriosis is a chronic inflammatory disease. Endometriotic cysts contain hemoglobin (Hb) and infiltrated macrophages, indicating that the metabolism of Hb by macrophages may play an important role in the inflammation of endometriotic cysts. In this study, we investigated the distribution of immune cells and CD163 (Hb receptor)-positive cells in the endometriotic cyst wall using immunohistochemistry. We also examined the role of macrophage activation by Hb on the pathogenesis of endometriotic cysts by measuring the cytokine concentration in the cystic fluids and macrophage-culture supernatant using ELISA. Macrophages were the most prominent immune cells observed in the endometriotic cysts and were differentially distributed in the different histological areas of the cyst wall. The localization of CD163-positive macrophages was restricted to the hemorrhagic and outer areas in the cyst wall. High concentrations of IL-6 and CCL2 were found in the cystic fluids, and inflammatory cytokines (IL-6, TNF-α, and CCL2) were secreted from macrophages on stimulation by Hb. IL-6 is a promotional factor for endometriotic stromal cells and ovarian clear cell carcinoma, the most common histological subtype of endometriosis-related ovarian cancer, hence, the continuous activation of macrophages by Hb could be a potential mechanism underlying endometriosis development and carcinogenesis.





Similar content being viewed by others
References
Zondervan KT, Becker CM, Missmer SA (2020) Endometriosis. N Engl J Med 382:1244–1256
Irving JA, Clement PB (2019) Disease of the peritoneum. In: Kurman RJ, Ronnett BM, Ellenson L (eds) Blaustein’s pathology of the female genital tract, 7th edn. Springer Nature Switzerland AG, Switzerland, pp 771–840
Nisolle M, Donnez J (1997) Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of rectovaginal septum are three different entities. Fertil Steril 68:585–596
Adghari S, Valizadeh A, Aghebati-Maleki L, Nouri M, Yousefi M (2018) Endometriosis: perspective, lights, and shadows of etiology. Biomed Pharmacother 106:163–174
Vinatier D, Dufour P, Oosterlynck D (1996) Immunological aspects of endometriosis. Hum Reprod Update 2:371–384
Oral E, Olive DL, Arici A (1996) The peritoneal environment in endometriosis. Hum Reprod Update 2:385–398
Kobayashi H, Higashiura Y, Shigetomi H, Kajihara H (2014) Pathogenesis of endometriosis: the role of initial infection and subsequent sterile inflammation. Mol Med Rep 9:9–15
Sanchez AM, Vigano P, Somigliana E, Panina-Bordignon P, Vercellini P, Candiani M (2014) The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum Reprod Update 20:217–230
Fukumatsu Y, Katabuchi H, Miyamura S, Matsuura K, Okamura H, Naito M, Takahashi K (1992) Activated macrophages in the peritoneal fluid of women with endometriosis: examination of the intracytoplasmic localization of endogenous peroxidase and interleukin-1. Acta Obstet Gynaecol Jpn 44:529–536
Koyama N, Matsuura K, Okamura H (1993) Cytokines in the peritoneal fluid of patients with endometriosis. Int J Gynecol Obstet 43:45–50
Yih S, Katabuchi H, Araki M, Matsuura K, Takeya M, Takahashi K, Okamura H (2001) Expression of monocyte chemoattractant protein-1in peritoneal endometriotic cells. Virchows Arch 438:70–77
Okamura H, Katabuchi H, Kanzaki H (2002) Macrophages in reproductive biology. In: Burke B, Lewis CE (eds) The macrophage, 2nd edn. Oxford University Press, New York, pp 549–576
Khan KN, Kitajima M, Hiraki K, Fujishita A, Sekine I, Ishimaru T, Masuzaki H (2008) Immunopathogenesis of pelvic endometriosis: role of hepatocyte growth factor, macrophages and ovarian steroids. Am J Reprod Immunol 60:383–404
Itoh F, Komohara Y, Takaishi K, Honda R, Tashiro H, Kyo S, Katabuchi H, Takeya M (2013) Possible involvement of signal transducer and activator of transcription-3 in cell-cell interactions of peritoneal macrophages and endometrial stromal cells in human endometriosis. Fertil Steril 99:1705–1713
Ahn SH, Monsanto SP, Miller C, Singh SS, Thomas R, Tayade C (2015) Pathophysiology and immune dysfunction in endometriosis. Biomed Res Int 2015:795976
Riccio LGC, Santulli P, Marcellin L, Abrão MS, Batteux F, Chapron C (2018) Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol 50:39–49
Badawy SZ, Cuenca V, Kumar S, Holland J (1998) Effects of chocolate cyst fluid on endometrioma cell growth in culture. Fertil Steril 70:827–830
Fasciani A, D’Ambrogio G, Bocci G, Luisi S, Artini PG, Genazzani AR (2001) Vascular endothelial growth factor and interleukin-8 in ovarian cystic pathology. Fertil Steril 75:1218–1221
Daraï E, Detchev R, Hugol D, Quang NT (2003) Serum and cyst fluid levels of interleukin (IL)-6, IL-8 and tumour necrosis factor-alpha in women with endometriomas and benign and malignant cystic ovarian tumours. Hum Reprod 18:1681–1685
Velasco I, Aciéna P, Campos A, Aciéna MI, Ruiz-Maciá E (2010) Interleukin-6 and other soluble factors in peritoneal fluid and endometriomas and their relation to pain and aromatase expression. J Reprod Immunol 84:199–205
Iba Y, Harada T, Horie S, Deura I, Iwabe T, Terakawa N (2004) Lipopolysaccharide-promoted proliferation of endometriotic stromal cells via induction of tumor necrosis factor and interleukin-8 expression. Fertil Steril 82:1036–1042
Suen JL, Chang Y, Chiu PR, Hsieh TH, His E, Chen YC, Chen YF, Tsai Serum EM (2014) Level of IL-10 is increased in patients with endometriosis, and IL-10 promotes the growth of lesions in a murine model. Am J Pathol 184:464–471
Miller JE, Ahn SH, Monsanto SP, Khalaj K, Koti M, Tayade C (2017) Implications of immune dysfunction on endometriosis associated infertility. Oncotarget 8:7138–7147
Munksgaard PS, Blaakaer J (2012) The association between endometriosis and ovarian cancer: a review of histological, genetic and molecular alterations. Gynecol Oncol 124:164–169
Pavone ME, Lyttle BM (2015) Endometriosis and ovarian cancer: links, risks, and challenges faced. Int J Womens Health 7:663–672
Yanaihara N, Hirata Y, Yamaguchi N, Noguchi Y, Saito M, Nagata C, Takakura S, Yamada K, Okamoto A (2016) Antitumor effects of interleukin-6 (IL-6)/interleukin-6 receptor (IL-6R) signaling pathway inhibition in clear cell carcinoma of the ovary. Mol Carcinog 55:832–841
Nanda A, Thangapandi K, Banerjee P, Dutta M, Wangdi T, Sharma P, Chaudhury K, Jana SK (2020) Cytokines, angiogenesis, and extracellular matrix degradation are augmented by oxidative stress in endometriosis. Ann Lab Med 40:390–397
Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK (2001) Identification of the haemoglobin scavenger receptor. Nature 409:198–201
Bacci M, Capobianco A, Monno A, Cottone L, Puppo FD, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S, Panina-Bordignon P, Manfredi AA, Rovere-Querini P (2009) Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol 175:547–556
Acknowledgements
We thank Ms. Hikaru Sakamato, Mr. Takuto Tsuru, Ms. Ayaka Nakashima, Mr. Takenobu Nakagawa, and all members of the Department of Cell Pathology and the Department of Obstetrics and Gynecology, Kumamoto University for their technical assistance. We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kusunoki, M., Fujiwara, Y., Komohara, Y. et al. Hemoglobin-induced continuous activation of macrophages in endometriotic cysts: a potential mechanism of endometriosis development and carcinogenesis. Med Mol Morphol 54, 122–132 (2021). https://doi.org/10.1007/s00795-020-00272-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-020-00272-4