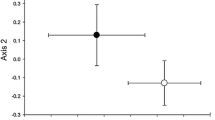
Abstract
Despite abounding evidence that leaf litter traits can predict decomposition rate, the way these traits influence trophic efficiency and element transfer to higher trophic levels is not resolved. Here, we used litter labeled with 13C and 15N stable isotopes to trace fluxes of litter C and N from four leaf types to freshwater invertebrate communities. We measured absolute (mg C or N) and relative assimilation (percentage of litter C or N incorporated into invertebrate biomass relative to C and N lost during decomposition). Four patterns emerged: (1) Invertebrate communities assimilated more C and N from slowly decomposing litter than communities feeding on rapidly decomposing litter; (2) absolute assimilation of both C and N in leaf packs was positively correlated with the relative biomass of invertebrate taxa in leaf packs; (3) Chironomidae larvae, which colonize packs in the early decomposition stages, assimilated the most C and N by the end of the 35-day experiment; and (4) most taxa, spanning five functional feeding groups (collector–gatherers, shredders, collector–filterers, scrapers, and predators), showed similar patterns in both absolute and relative assimilation across leaf types. These results challenge traditional views of litter quality by demonstrating that trophic efficiency is negatively associated with decomposition rate across these four leaf types.






Similar content being viewed by others
References
Alonso A, González-Muñoz N, Castro-Díez P. 2010. Comparison of leaf decomposition and macroinvertebrate colonization between exotic and native trees in a freshwater ecosystem. Ecol Res 25:647–53.
Anderson NH, Lehmkuhl DM. 1968. Catastrophic drift of insects in a woodland stream. Ecology 49:198–206.
Barlöcher F. 1980. Leaf-eating invertebrates as competitors of aquatic hyphomycetes. Oecologia 47:303–6.
Benke AC. 1998. Production dynamics of riverine chironomids: extremely high biomass turnover rates of primary consumers. Ecology 79:899–910.
Benke AC, Huryn AD, Smock LA, Wallace JB. 1999. Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. J N Am Benthol Soc 18:308–43.
Brittain JE, Eikeland TJ. 1988. Invertebrate drift—a review. Hydrobiologia 166:77–93.
Canhoto C, Graça MAS. 1995. Food value of introduced eucalypt leaves for a Mediterranean stream detritivore: Tipula lateralis. Freshw Biol 34:209–14.
Cheever BM, Webster JR, Bilger EE, Thomas SA. 2013. The relative importance of exogenous and substrate-derived nitrogen for microbial growth during leaf decomposition. Ecology 94:1614–25.
Chung N, Suberkropp K. 2009. Contribution of fungal biomass to the growth of the shredder, Pycnopsyche gentilis (Trichoptera: Limnephilidae). Freshw Biol 54:2212–24.
Compson ZG, Adams KJ, Edwards JA, Maestas JM, Whitham TG, Marks JC. 2013. Leaf litter quality affects aquatic insect emergence: contrasting patterns from two foundation trees. Oecologia 173:507–19.
Compson ZG, Hungate BA, Koch GW, Hart SC, Maestas JM, Adams KJ, Whitham TG, Marks JC. 2015. Closely related tree species differentially influence the transfer of carbon and nitrogen from leaf litter up the aquatic food web. Ecosystems 18:186–201.
Compson ZG, Hungate BA, Whitham TG, Meneses N, Busby PE, Wojtowicz T, Ford AC, Adams KJ, Marks JC. 2016. Plant genotype influences aquatic-terrestrial ecosystem linkages through timing and composition of insect emergence. Ecosphere 7:e01331. https://doi.org/10.1002/ecs2.1331.
Compson ZG, Hungate BA, Whitham TG, Koch GW, Dijkstra P, Siders AC, Wojtowicz T, Jacobs R, Rakestraw DN, Allred KE, Sayer CK. 2018. Linking tree genetics and stream consumers: isotopic tracers elucidate controls on carbon and nitrogen assimilation. Ecology 99:1759–70.
Cornwell WK, Cornelissen JH, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N, Quested HM. 2008. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–71.
Cross WF, Johnson BR, Wallace JB, Rosemond AD. 2005. Ecological stoichiometry in freshwater benthic systems: recent progress and perspectives. Limnol Oceanogr 50:1730–9.
Cross WF, Wallace JB, Rosemond AD, Eggert SL. 2006. Whole-system nutrient enrichment increases secondary production in a detritus-based ecosystem. Ecology 87:1556–65.
Demi LM, Benstead JP, Rosemond AD, Maerz JC. 2018. Litter P content drives consumer production in detritus-based streams spanning an experimental N: P gradient. Ecology 99:347–59.
Dickman EM, Newell JM, González MJ, Vanni MJ. 2008. Light, nutrients, and food-chain length constrain planktonic energy transfer efficiency across multiple trophic levels. Proc Natl Acad Sci 105:18408–12.
Driebe EM, Whitham TG. 2000. Cottonwood hybridization affects tannin and nitrogen content of leaf litter and alters decomposition. Oecologia 123:99–107.
Evans-White MA, Halvorson HM. 2017. Comparing the ecological stoichiometry in green and brown food webs—a review and meta-analysis of freshwater food webs. Front Microbiol 8:1184.
Fioretto A, Di Nardo C, Papa S, Fuggi A. 2005. Lignin and cellulose degradation and nitrogen dynamics during decomposition of three leaf litter species in a Mediterranean ecosystem. Soil Biol Biochem 37:1083–91.
Fogelman KJ, Bilger MD, Holt JR, Matlaga DP. 2018. Decomposition and benthic macroinvertebrate communities of exotic Japanese knotweed (Fallopia japonica) and American sycamore (Platanus occidentalus) detritus within the Susquehanna River. J Freshw Ecol 33:299–310.
Fugère V, Andino P, Espinosa R, Anthelme F, Jacobsen D, Dangles O. 2012. Testing the stress-gradient hypothesis with aquatic detritivorous invertebrates: insights for biodiversity-ecosystem functioning research. J Anim Ecol 81:1259–67.
Fuller CL, Evans-White MA, Entrekin SA. 2015. Growth and stoichiometry of a common aquatic detritivore respond to changes in resource stoichiometry. Oecologia 117:837–48.
Gessner MO, Chauvet E. 1994. Importance of stream microfungi in controlling breakdown rates of leaf litter. Ecology 75:1807–17.
Golladay S, Webster J, Benfield EF. 1983. Factors affecting food utilization by a leaf shredding aquatic insect: leaf species and conditioning time. Ecography 6:157–62.
Gonçalves JF, Graça MAS, Callisto M. 2006. Leaf-litter breakdown in 3 streams in temperate, Mediterranean, and tropical Cerrado climates. J N Am Benthol Soc 25:344–55.
Graça MAS. 2001. The role of invertebrates on leaf litter decomposition in streams—a review. Int Rev Hydrobiol 86:383–93.
Graça MAS, Cressa C, Gessner MO, Feio MJ, Callies KA, Barrios C. 2001. Food quality, feeding preferences, survival and growth of shredders from temperate and tropical streams. Freshw Biol 46:947–57.
Grubbs SA, Cummins KW. 1994. Processing and macroinvertebrate colonization of black cherry (Prunus serotina) leaves in two streams differing in summer biota, thermal regime and riparian vegetation. Am Midl Nat 132:284–93.
Halvorson HM, Scott JT, Sanders AJ, Evans-White MA. 2015. A stream insect detritivore violates common assumptions of threshold elemental ratio bioenergetics models. Freshw Sci 34:508–18.
Halvorson HM, Sperfeld E, Evans-White MA. 2017. Quantity and quality limit detritivore growth: mechanisms revealed by ecological stoichiometry and co-limitation theory. Ecology 93:2995–3002.
Hladyz S, Gessner MO, Giller PS, Pozo J, Woodward G. 2009. Resource quality and stoichiometric constraints on stream ecosystem functioning. Freshw Biol 54:957–70.
Hirabayashi K, Wotton RS. 1998. Organic matter processing by chironomid larvae (Diptera: Chironomidae). Hydrobiologia 382:151–9.
Humphreys WF. 1979. Production and respiration in animal populations. J Anim Ecol 48:427–53.
Hutchens JJ, Benfield EF, Webster JR. 1997. Diet and growth of a leaf-shredding caddisfly in southern Appalachian streams of contrasting disturbance history. Hydrobiologia 346:193–201.
Kinzig AP, Harte J. 1998. Selection of micro-organisms in a spatially explicit environment and implications for plant access to nitrogen. J Ecol 86:841–53.
Kohlmeier S, Smits TH, Ford RM, Keel C, Harms H, Wick LY. 2005. Taking the fungal highway: mobilization of pollutant-degrading bacteria by fungi. Environ Sci Technol 39:4640–6.
Kominoski JS, Larranaga S, Richardson JS. 2012. Invertebrate feeding and emergence timing vary among streams along a gradient of riparian forest composition. Freshw Biol 57:759–72.
Lake PS, Doeg TJ. 1985. Macroinvertebrate colonization of stones in two upland southern Australian streams. Hydrobiologia 126:199–211.
LeRoy CJ, Marks JC. 2006. Litter quality, stream characteristics and litter diversity influence decomposition rates and macroinvertebrates. Freshw Biol 51:605–17.
LeRoy CJ, Whitham TG, Wooley SC, Marks JC. 2007. Within-species variation in foliar chemistry influences leaf-litter decomposition in a Utah river. J N Am Benthol Soc 26:426–38.
Lindeman RL. 1942. The trophic-dynamic aspect of ecology. Ecology 23:399–417.
Makkonen M, Berg MP, Handa IT, Hättenschwiler S, van Ruijven J, van Bodegom PM, Aerts R. 2012. Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol Lett 15:1033–41.
Manning DWP, Rosemond AD, Gulis V, Benstead JP, Kominoski JS, Maerz JC. 2016. Convergence of detrital stoichiometry predicts thresholds of nutrient-stimulated breakdown in streams. Ecol Appl 26:1745–57.
Marcarelli AM, Baxter CV, Mineau MM, Hall RO. 2011. Quantity and quality: unifying food web and ecosystem perspectives on the role of resource subsidies in freshwaters. Ecology 92:1215–25.
Marks JC. 2019. Revisiting the fates of dead leaves that fall into streams. Annu Rev Ecol Evolut Syst 50:547–68.
McCune B, Grace JB, Urban DL. 2002. Analysis of ecological communities. Gleneden Beach (OR): MjM Software Design.
McCune B, Mefford MJ. 2011. PC-ORD. v. 6. Multivariate analysis of ecological data. Gleneden Beach (OR): MjM Software.
McDowell WH, Fisher SG. 1976. Autumnal processing of dissolved organic matter in a small woodland stream ecosystem. Ecology 57:561–9.
Melillo JM, Aber JD, Muratore JF. 1982. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–6.
Merritt RW, Cummins KW, Berg MB. 2008. An introduction to the aquatic insects of North America. Dubuque (IA): Kendall Hunt.
Meyer JL. 1994. The microbial loop in flowing waters. Microb Ecol 28:195–9.
Minshall GW, Petersen RC, Nimz CF. 1985. Species richness in streams of different size from the same drainage basin. Am Nat 125:16–38.
Motomori K, Mitsuhashi H, Nakano S. 2001. Influence of leaf litter quality on the colonization and consumption of stream invertebrate shredders. Ecol Res 16:173–82.
Norman BC, Whiles MR, Collins SM, Flecker AS, Hamilton SK, Johnson SL, Rosi EJ, Ashkenas LR, Bowden WB, Crenshaw CL, Crowl T, Dodds WK, Hall RO, El-Sabaawi R, Griffiths NA, Marti E, McDowell WH, Peterson SD, Rantala HM, Riis T, Simon KS, Tank JL, Thomas SA, von Schiller D, Webster JR. 2017. Drivers of nitrogen transfer in stream food webs across continents. Ecology 98:3044–55.
Ostrofsky ML. 1997. Relationship between chemical characteristics of autumn-shed leaves and aquatic processing rates. J N Am Benthol Soc 16:750–9.
Pastor A, Compson ZG, Dijkstra P, Riera JL, Martí E, Sabater F, Hungate BA, Marks JC. 2014. Stream carbon and nitrogen supplements during leaf litter decomposition: contrasting patterns for two foundation species. Oecologia 176:1111–21.
Peckarsky BL. 1986. Colonization of natural substrates by stream benthos. Can J Fish Aquat Sci 43:700–9.
Perry WB, Benfield EF, Perry SA, Webster JR. 1987. Energetics, growth, and production of a leaf-shredding stonefly in an Appalachian mountain stream. J N Am Benthol Soc 6:12–25.
Petersen RC, Cummins KW. 1974. Leaf processing in a woodland stream. Freshw Biol 4:343–68.
Rahman MM, Tsukamoto J, Rahman MM, Yoneyama A, Mostafa KM. 2013. Lignin and its effects on litter decomposition in forest ecosystems. Chem Ecol 29:540–53.
Rosemond AD, Benstead JP, Bumpers PM, Gulis V, Kominoski JS, Manning DWP, Suberkropp K, Wallace JB. 2015. Experimental nutrient additions accelerate terrestrial carbon loss from stream ecosystems. Science 347:1142–5.
Romito AM, Eggert SL, Diez JM, Wallace JB. 2010. Effects of seasonality and resource limitation on organic matter turnover by Chironomidae (Diptera) in southern Appalachian headwater streams. Limnol Oceanogr 55:1083–92.
Siders AC, Compson ZG, Hungate BA, Dijkstra P, Koch GW, Wymore AS, Grandy AS, Marks JC. 2018. Litter identity affects assimilation of carbon and nitrogen by a shredding caddisfly. Ecosphere 9:e02340. https://doi.org/10.1002/ecs2.2340.
Spain AV, Le Feuvre RP. 1987. Breakdown of four litters of contrasting quality in a tropical Australian rainforest. J Appl Ecol 24:279–88.
Steffan SA, Chikaraishi Y, Currie CR, Horn H, Gaines-Day HR, Pauli JN, Zalapa JE, Ohkouchi N. 2015. Microbes are trophic analogs of animals. Proc Natl Acad Sci 112:15119–24.
Steffan SA, Chikaraishi Y, Dharampal PS, Pauli JN, Guédot C, Ohkouchi N. 2017. Unpacking brown food-webs: animal trophic identity reflects rampant microbivory. Ecol Evolut 7:3532–41.
Suberkropp K. 1992. Interactions with invertebrates. Barlöcher F, Ed. The ecology of aquatic hyphomycetes. Berlin: Springer. pp 118–31.
Triska FJ, Sedell JR. 1976. Decomposition of four species of leaf litter in response to nitrate manipulation. Ecology 57:783–92.
Vadeboncoeur Y, Power ME. 2017. Attached algae: the cryptic base of inverted trophic pyramids in freshwaters. Annu Rev Ecol Evolut Syst 48:255–79.
Webster JR, Waide JB. 1982. Effects of forest clearcutting on leaf breakdown in a southern Appalachian stream. Freshw Biol 12:331–44.
Webster JR, Benfield EF. 1986. Vascular plant breakdown in freshwater ecosystems. Annu Rev Ecol Syst 17:567–94.
Wise DH, Molles MC. 1979. Colonization of artificial substrates by stream insects: influence of substrate size and diversity. Hydrobiologia 65:69–74.
Wymore AS, Compson ZG, McDowell WG, Potter JD, Hungate BA, Whitham TG, Marks JC. 2015. Leaf-litter leachate is distinct in optical properties and bioavailability to stream heterotrophs. Freshw Sci 34:857–66.
Acknowledgements
We thank Phil Patterson from the NAU Research Greenhouse for assisting with growing and labeling plants. We thank Greg Florian for helping to build growth chambers. We are appreciative of Tom Kaminski, Jesse Maestas, David Green, Raemy Winton, David Rakestraw, Jordan Pletzer, Shannon Hagerty, Bri Finley, Janice Talley, Adriana Nimer, and Rosie Alling for help in the field and laboratory. We appreciate useful discussions with Mike Rotter, Sean Mahoney, and Danelle Larson. We thank members of the Center for Ecosystem Science and Society, at Northern Arizona University, for their valuable insights and feedback throughout the design of the field experiment and development of the manuscript. We appreciate the feedback from the editor and two anonymous reviewers, which substantially improved the manuscript. Funding was provided through NSF Grants DEB-1120343 and DEB-1655357.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
ACS, ZGC, BAH, PD, GWK, and JCM designed the study. ACS and ZGC grew and labeled the leaves and conducted the field experiment. ACS wrote the first draft of the manuscript and ZGC, BAH, GWK, and JCM made substantial contributions to revising the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Siders, A.C., Compson, Z.G., Hungate, B.A. et al. The Influence of Leaf Type on Carbon and Nitrogen Assimilation by Aquatic Invertebrate Communities: A New Perspective on Trophic Efficiency. Ecosystems 24, 788–805 (2021). https://doi.org/10.1007/s10021-020-00550-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-020-00550-3