Abstract.
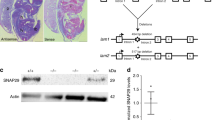
We report a novel spontaneous mutation named nax in mice, which exhibit delayed hair appearance and ataxia in a homozygote state. Histological analyses of nax brain revealed an overall impairment of the cerebellar cortex. The classical cortical cytoarchitecture was disrupted, the inner granule cell layer was not obvious, the Purkinje cells were not aligned as a Purkinje cell layer, and Bergmann glias did not span the molecular layer. Furthermore, histological analyses of skin showed that the hair follicles were also abnormal. We mapped the nax locus between marker D2Mit158 and D2Mit100 within a region of 800 kb in the middle of chromosome 2 and identified a missense mutation (Gly244Glu) in Acp2, a lysosomal monoesterase. The Glu244 mutation does not affect the stability of the Acp2 transcript, however it renders the enzyme inactive. Ultrastructural analysis of nax cerebellum showed lysosomal storage bodies in nucleated cells, suggesting progressive degeneration as the underlying mechanism. Identification of Acp2 as the gene mutated in nax mice provides a valuable model system for studying the role of Acp2 in cerebellum and skin homeostasis.






Similar content being viewed by others
References
Eskelinen EL, Tanaka Y, Saftig P (2003) At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol 13:137–145
Gieselmann V (1995) Lysosomal storage diseases. Biochim Biophys Acta 1270:103–136
Drexler HG, Gignac SM (1994) Characterization and expression of tartrate-resistant acid phosphatase (TRAP) in hematopoietic cells. Leukemia 8:359–368
Gieselmann V, Hasilik A, Figura K von (1984) Tartrate-inhibitable acid phosphatase. Purification from placenta, characterization and subcellular distribution in fibroblasts. Hoppe Seyler Z Physiol Chem 365:651–660
Clark SA, Ambrose WW, Anderson TR, Terrell RS, Toverud SU (1989) Ultrastructural localization of tartrate-resistant, purple acid phosphatase in rat osteoclasts by histochemistry and immunocytochemistry. J Bone Miner Res 4:399–405
De Duve C (1959) Subcellular particles. In: Hayashi T (ed) Ronald Press, pp 128–159
Geier C, Kreysing J, Boettcher H, Pohlmann R, Figura K von (1992) Localization of lysosomal acid phosphatase mRNA in mouse tissues. J Histochem Cytochem 40:1275–1282
Hille A, Klumperman J, Geuze HJ, Peters C, Brodsky, FM, Figura K von (1992) Lysosomal acid phosphatase is internalized via clathrin-coated pits. Eur J Cell Biol 59:106–115
Braun M, Waheed A, Figura K von (1989) Lysosomal acid phosphatase is transported to lysosomes via the cell surface. EMBO J 8:3633–3640
Gottschalk S, Waheed A, Schmidt B, Laidler P, Figura K von, (1989) Sequential processing of lysosomal acid phosphatase by a cytoplasmic thiol proteinase and a lysosomal aspartyl proteinase. EMBO J 8:3215–3219
Saftig P, Hartmann D, Lüllmann-Rauch R, Wolff J, Evers M, Köster A, Hetman M, Figura K von, Peters C (1997) Mice deficient in lysosomal acid phosphatase develop lysosomal storage in the kidney and central nervous system. J Biol Chem 272:18628–18634
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Waheed A, Van Etten RL, Gieselmann V, Figura K von (1985) Immunological characterization of human acid phosphatase gene products. Biochem Genet 23:309–319
Rickmann M, Wolff JR (1995) S100 protein expression in subpopulations of neurons of rat brain. Neuroscience 67:977–991
Mannan AU, Nayernia K, Mueller C, Burfeind P, Adham IM, Engel W (2003) Male mice lacking the Theg (testicular haploid expressed gene) protein undergo normal spermatogenesis and are fertile. Biol Reprod 69:788–796
Hatten ME, Heintz N (1995) Mechanisms of neural patterning and specification in the developing cerebellum. Annu Rev Neurosci 18:385–408
Hatten ME (1990) Riding the glial monorail: a common mechanism for glial-guided neuronal migration in different regions of the developing mammalian brain. Trends Neurosci 13:179–184
Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, Hofmann U, Foitzik K, Mecklenburg L, Handjiski B (1999) A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol 113:523–532
Jakob CG, Lewinski K, Kuciel R, Ostrowski W, Lebioda L (2000) Crystal structure of human prostatic acid phosphatase. Prostate 42:211–218
Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna I, Pant HC, Brady RO, Martin LJ, Kulkarni AB (1996) Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci U S A 93:11173–11178
Hess EJ (1996) Identification of the weaver mouse mutation: the end of the beginning. Neuron 16:1073–1076
Miyata T, Nakajima K, Mikoshiba K, Ogawa M (1997) Regulation of Purkinje cell alignment by reelin as revealed with CR-50 antibody. J Neurosci 17:3599–3609
Goldowitz D, Cushing RC, Laywell E, D’Arcangelo G, Sheldon M, Sweet HO, Davisson M, Steindler D, Curran T, (1997) Cerebellar disorganization characteristic of reeler in scrambler mutant mice despite presence of reelin. J Neurosci 17:8767–8777
Nakamura M, Sundberg JP, Paus R (2001) Mutant laboratory mice with abnormalities in hair follicle morphogenesis, cycling, and/or structure: annotated tables. Exp Dermatol 10:369–390
Roth W, Deussing J, Botchkarev VA, Pauly-Evers M, Saftig P, Hafner A, Schmidt P, Schmahl W, Scherer J, Anton-Lamprecht I, Figura K von, Paus R, Peters C (2000) Cathepsin L deficiency as molecular defect of furless: hyperproliferation of keratinocytes and pertubation of hair follicle cycling. FASEB J 14:2075–2086
Suter A, Everts V, Boyde A, Jones SJ, Lullmann-Rauch R, Hartmann D, Hayman AR, Cox TM, Evans MJ, Meister T, Figura K von, Saftig P (2001) Overlapping functions of lysosomal acid phosphatase (LAP) and tartrate-resistant acid phosphatase (Acp5) revealed by doubly deficient mice. Development 128:4899–4910
Fusek M, Vetvicka V (1994) Mitogenic function of human procathepsin D: the role of the propeptide. Biochem J 303:775–780
Vetvicka V, Vetvickova J, Fusek M (1998) Effect of procathepsin D and its activation peptide on prostate cancer cells. Cancer Lett 129:55–59
Bahr BA, Bendiske J (2002) The neuropathogenic contributions of lysosomal dysfunction. J Neurochem 83:481-489
Walkley SU (1998) Cellular pathology of lysosomal storage disorders. Brain Pathol 8:175–193
Munoz R MV, Santos AC, Graziadio C, Pina-Neto JM (1997) Cerebello-trigeminal-dermal dysplasia (Gomez-Lopez-Hernandez syndrome): description of three new cases and review. Am J Med Genet 72:34–39
Keeler LC, Marsh SE, Leeflang EP, Woods CG, Sztriha L, Al-Gazali L, Gururaj A, Gleeson JG (2003) Linkage analysis in families with Joubert syndrome plus oculo-renal involvement identifies the CORS2 locus on chromosome 11p12-q13.3. Am J Hum Genet 73:656–662
Valente EM, Salpietro DC, Brancati F, Bertini E, Galluccio T, Tortorella G, Briuglia S, Dallapiccola B (2003) Description, nomenclature, and mapping of a novel cerebello-renal syndrome with the molar tooth malformation. Am J Hum Genet 73:663–670
Ranum LP, Schut LJ, Lundgren JK, Orr HT, Livingston DM (1994) Spinocerebellar ataxia type 5 in a family descended from the grandparents of President Lincoln maps to chromosome 11. Nat Genet 8:280–284
Acknowledgements.
This work was funded by the Deutsche Forschungsgemeinschaft through the DFG-Research Center for Molecular Physiology of the Brain to W.E. and M.R. The authors would like to thank S. Zimmermann for operational help, M. Wahl for assistance with structural biology programs, and K. von Figura for useful discussions and helping us with Acp2 enzymatic assay. A. Eberwein, M. Moeschner, and M. Steckel for technical assistance, and our animal housekeepers for breeding and maintaining these mice.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Mannan, A.U., Roussa, E., Kraus, C. et al. Mutation in the gene encoding lysosomal acid phosphatase (Acp2) causes cerebellum and skin malformation in mouse. Neurogenetics 5, 229–238 (2004). https://doi.org/10.1007/s10048-004-0197-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-004-0197-9