Abstract
To evaluate the efficacy and safety of total glucosides of peony (TGP) in adults with primary Sjögren’s syndrome (pSS). A multi-center, randomized, double-blinded, placebo-controlled study was conducted between March 2012 and July 2014 at ten Chinese hospitals. In total, 320 pSS patients—classified according to the 2002 American-European Consensus Group Criteria—were randomized (2:1 ratio) to receive TGP(600 mg, tid) in the TGP group or placebo for 24 weeks in the placebo group. Study personnel, investigators, and patients were blinded to the treatment grouping. The primary endpoint was the improvement of EULAR Sjögren’s Syndrome Patient Reported Index (ESSPRI) at week 24. The secondary endpoints were dry eyes/mouth/skin/nose/throat/vagina visual analogue scale (VAS), pain and discomfort VAS, fatigue VAS, mental discomfort VAS, patient global assessment (PGA), EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI), Schirmer’s test, basal/stimulated salivary flow-rate values, and erythrocyte sedimentation rate (ESR). All adverse events were recorded during the trial period. ESSPRI improved more in the TGP than the placebo group (p < 0.001). Dry eyes/throat/vagina VAS, fatigue VAS, mental discomfort VAS, PGA, Schirmer’s test, and ESR also improved more in the TGP group than in the placebo group (all p < 0.05). Stimulated salivary flow-rate values increased in the TGP group at week 12 but not at week 24. Adverse events in TGP group were 10.9%. TGP can alleviate some dryness symptoms as well as disease activity in pSS patients over 24 weeks. TGP was well tolerated by study subjects. TGP seems to be an effective and safe treatment for pSS.




Similar content being viewed by others
Change history
03 December 2018
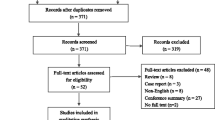
The authors regret that the Fig. 1 in the original version of this article contained an error. In the left column, 211 cases in the TGP group should be followed up for 8 weeks before 12 weeks. The correct figure presented in this article.
References
Youinou P, Pers JO (2015) Primary Sjogren's syndrome at a glance today. Joint Bone Spine 82(2):75–76. https://doi.org/10.1016/j.jbspin.2014.10.018
Ramos-Casals M, Tzioufas AG, Stone JH, Siso A, Bosch X (2010) Treatment of primary Sjogren syndrome: a systematic review. JAMA 304(4):452–460. https://doi.org/10.1001/jama.2010.1014
Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH (2002) Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 61(6):554–558
Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM (2009) Systemic and local interleukin-17 and linked cytokines associated with Sjogren's syndrome immunopathogenesis. Am J Pathol 175(3):1167–1177. https://doi.org/10.2353/ajpath.2009.090319
De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD, 3rd, Fang B, Zheng X, Ma P, Farley WJ, Siemasko KF, Niederkorn JY, Stern ME, Li DQ, Pflugfelder SC (2009) IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol 2 (3):243–253. doi:https://doi.org/10.1038/mi.2009.5
Lin X, Rui K, Deng J, Tian J, Wang X, Wang S, Ko KH, Jiao Z, Chan VS, Lau CS, Cao X, Lu L (2015) Th17 cells play a critical role in the development of experimental Sjogren's syndrome. Ann Rheum Dis 74(6):1302–1310. https://doi.org/10.1136/annrheumdis-2013-204584
Deveci H, Kobak S (2014) The efficacy of topical 0.05% cyclosporine A in patients with dry eye disease associated with Sjogren's syndrome. Int Ophthalmol 34(5):1043–1048. https://doi.org/10.1007/s10792-014-9901-4
Lin T, Gong L (2015) Topical fluorometholone treatment for ocular dryness in patients with Sjogren syndrome: a randomized clinical trial in China. Medicine (Baltimore) 94(7):e551. https://doi.org/10.1097/md.0000000000000551
Tanner K, Nissen SL, Merrill RM, Miner A, Channell RW, Miller KL, Elstad M, Kendall KA, Roy N (2015) Nebulized isotonic saline improves voice production in Sjogren's syndrome. Laryngoscope 125(10):2333–2340. https://doi.org/10.1002/lary.25239
Skopouli FN, Jagiello P, Tsifetaki N, Moutsopoulos HM (1996) Methotrexate in primary Sjogren's syndrome. Clin Exp Rheumatol 14(5):555–558
Gottenberg JE, Ravaud P, Puechal X, Le Guern V, Sibilia J, Goeb V, Larroche C, Dubost JJ, Rist S, Saraux A, Devauchelle-Pensec V, Morel J, Hayem G, Hatron P, Perdriger A, Sene D, Zarnitsky C, Batouche D, Furlan V, Benessiano J, Perrodeau E, Seror R, Mariette X (2014) Effects of hydroxychloroquine on symptomatic improvement in primary Sjogren syndrome: the JOQUER randomized clinical trial. JAMA 312(3):249–258. https://doi.org/10.1001/jama.2014.7682
Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, Berthelot JM, Perdriger A, Puechal X, Le Guern V, Sibilia J, Gottenberg JE, Chiche L, Hachulla E, Hatron PY, Goeb V, Hayem G, Morel J, Zarnitsky C, Dubost JJ, Pers JO, Nowak E, Saraux A (2014) Treatment of primary Sjogren syndrome with rituximab: a randomized trial. Ann Intern Med 160(4):233–242. https://doi.org/10.7326/m13-1085
van Woerkom JM, Kruize AA, Geenen R, van Roon EN, Goldschmeding R, Verstappen SM, van Roon JA, Bijlsma JW (2007) Safety and efficacy of leflunomide in primary Sjogren's syndrome: a phase II pilot study. Ann Rheum Dis 66(8):1026–1032. https://doi.org/10.1136/ard.2006.060905
Price EJ, Rigby SP, Clancy U, Venables PJ (1998) A double blind placebo controlled trial of azathioprine in the treatment of primary Sjogren's syndrome. J Rheumatol 25(5):896–899
Willeke P, Schluter B, Becker H, Schotte H, Domschke W, Gaubitz M (2007) Mycophenolate sodium treatment in patients with primary Sjogren syndrome: a pilot trial. Arthritis Res Ther 9(6):R115. https://doi.org/10.1186/ar2322
Atzeni F, Doria A, Turiel M, Sarzi-Puttini P (2007) What is the role of rituximab in the treatment of rheumatoid arthritis? Autoimmun Rev 6(8):553–558. https://doi.org/10.1016/j.autrev.2007.02.004
Meiners PM, Vissink A, Kroese FG, Spijkervet FK, Smitt-Kamminga NS, Abdulahad WH, Bulthuis-Kuiper J, Brouwer E, Arends S, Bootsma H (2014) Abatacept treatment reduces disease activity in early primary Sjogren's syndrome (open-label proof of concept ASAP study). Ann Rheum Dis 73(7):1393–1396. https://doi.org/10.1136/annrheumdis-2013-204653
De Vita S, Quartuccio L, Seror R, Salvin S, Ravaud P, Fabris M, Nocturne G, Gandolfo S, Isola M, Mariette X (2015) Efficacy and safety of belimumab given for 12 months in primary Sjogren's syndrome: the BELISS open-label phase II study. Rheumatology (Oxford) 54 (12):2249–2256. doi:https://doi.org/10.1093/rheumatology/kev257
He DY, Dai SM (2011) Anti-inflammatory and immunomodulatory effects of paeonia lactiflora pall., a traditional chinese herbal medicine. Front Pharmacol 2:10. https://doi.org/10.3389/fphar.2011.00010
Zhou Z, Lin J, Huo R, Huang W, Zhang J, Wang L, Sun Y, Shen B, Li N (2012) Total glucosides of paeony attenuated functional maturation of dendritic cells via blocking TLR4/5 signaling in vivo. Int Immunopharmacol 14(3):275–282. https://doi.org/10.1016/j.intimp.2012.07.012
Lin J, Xiao L, Ouyang G, Shen Y, Huo R, Zhou Z, Sun Y, Zhu X, Zhang J, Shen B, Li N (2012) Total glucosides of paeony inhibits Th1/Th17 cells via decreasing dendritic cells activation in rheumatoid arthritis. Cell Immunol 280(2):156–163. https://doi.org/10.1016/j.cellimm.2012.12.005
Li CL, He J, Li ZG, Zheng LW, H. H (2013) Effects of total glucosides of paeony for delaying onset of Sjogren's syndrome: an animal study. J Craniomaxillofac Surg 41 (7):610–615. doi:https://doi.org/10.1016/j.jcms.2012.11.042
Wu GL, Pu XH, Yu GY, Li TY (2015) Effects of total glucosides of peony on AQP-5 and its mRNA expression in submandibular glands of NOD mice with Sjogren's syndrome. Eur Rev Med Pharmacol Sci 19(1):173–178
Li XM, Li XP, Wang GS, Qian L, Wang W (2006) Effectiveness and safety of total glucosides of peony in the treatment of patients with Sjögren syndrome. Anhui Medical Journal 27:370–371
Zhang HF, Hou P, Xiao WG (2007) Clinical observation on effect of total glucosides of paeony in treating patients with non-systemic involved Sjogren syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi 27(7):596–598
Zhou Y, Jin L, Kong F, Zhang H, Fang X, Chen Z, Wang G, Li X, Li X (2016) Clinical and immunological consequences of total glucosides of paeony treatment in Sjogren's syndrome: a randomized controlled pilot trial. Int Immunopharmacol 39:314–319. https://doi.org/10.1016/j.intimp.2016.08.006
Barone F, Colafrancesco S (2016) Sjogren's syndrome: from pathogenesis to novel therapeutic targets. Clin Exp Rheumatol 34(4 Suppl 98):58–62
Lu J, Yang PT, Shen H, Xiao WG, Zhao LJ (2006) Clinical application of total glucosides of paeony in Sjogren syndrome. J Chin Med Univ 35(5):522–524
Acknowledgments
None.
Source(s) of support
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
All authors declare that they have no conflicts of interest.
Electronic supplementary material
Supplementary Figure 1
Difference from baseline in dry skin VAS, dry nose VAS, dry throat VAS, dry vagina VAS, pain discomfort VAS, mental discomfort VAS at weeks 4, 8, 12, 18, and 24. p < 0.01 is considered significant after Bonferroni adjustment between TGP and placebo groups (PNG 207 kb)
Rights and permissions
About this article
Cite this article
Liu, X., Li, X., Li, X. et al. The efficacy and safety of total glucosides of peony in the treatment of primary Sjögren’s syndrome: a multi-center, randomized, double-blinded, placebo-controlled clinical trial. Clin Rheumatol 38, 657–664 (2019). https://doi.org/10.1007/s10067-018-4315-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4315-8