Abstract
Objectives
The Turkish population is vaccinated with Bacille Calmette-Guérin (BCG), and the BCG vaccination decreases the specificity of the tuberculin skin test (TST). The purpose of this study was to investigate the incidence of active tuberculosis (TBC) among rheumatic patients who were screened only with the QuantiFERON®-TB Gold In-Tube (QFT-GIT) test for latent TBC prior to biological treatment.
Methods
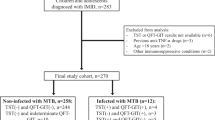
The Hacettepe University Biological Database (HUR-BIO) was used for latent TBC assessment. Consecutive patients were evaluated from July 2015 to October 2016 by a questionnaire that included the patients’ demographic characteristics, treatment history, and symptoms of active TBC. A total of 664 patients were interviewed by physicians. TBC statuses of the 671 non-interviewed patients were checked from the Turkish National Tuberculosis Registry records. Mean TBC incidence per year was calculated for anti-tumor necrosis factor-alpha (TNF-α) agents.
Results
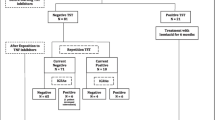
A total of 1335 (58.2% female) patients with the mean age of 44.2 ± 12.9 years were included. Of the patients, 836 (62.6%) had spondyloarthropathy, 432 (32.4%) had rheumatoid arthritis, and 67 (5%) had other rheumatologic diseases. The total biological drug exposure was 2292 patient-years (2043 patient-years for anti-TNF-α, 249 patient-years for non-TNF-α inhibitors). Positive and indeterminate QFT-GIT results were found in 258 (19.3%) and 23 (1.7%) patients, respectively. The median follow-up time after the onset of biological agent was 19.4 months (IQR = 29.5). Pulmonary TBC was found in 3 (0.2%) of the 1335 patients. The annual incidence of TBC was 147/100,000 patient-years for all TNF-α inhibitors (249/100,000 and 123/100,000 patient-years for QFT-GIT-positive and negative patients, respectively).
Conclusions
TBC incidence increased by nearly seven times the Turkish national TBC incidence. The QFT-GIT Test appears acceptable to determine latent TBC before biological agent use. Consequently, the QFT-GIT Test can be appropriately used in BCG-vaccinated countries.
Key Points • Our study contributes to filling the gap in the literature by reflecting real-life data about TBC frequency after QFT-GIT use in patients receiving biological agents. • The frequency of active TBC will remain within acceptable limits when only QFT-GIT is used in the screening of latent TBC prior to the use of biological agents in a population where the majority are vaccinated with BCG. • Using the QFT-GIT alone for latent TBC screening prior to biologic treatment in countries with high BCG vaccination rates reduces the number of patients needing isoniazid (INH) treatment. |

Similar content being viewed by others
References
Gomez-Reino JJ, Carmona L, Valverde VR et al (2003) Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum 48(8):2122–2127. https://doi.org/10.1002/art.11137
Denkinger CM, Dheda K, Pai M (2100) Guidelines on interferon-gamma release assays for tuberculosis infection: concordance, discordance or confusion? Clin Microbiol Infect 17(6):806–814. https://doi.org/10.1111/j.1469-0691.2011.03555.x
Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, Moreland LW, O’Dell J, Winthrop KL, Beukelman T, Bridges SL Jr, Chatham WW, Paulus HE, Suarez-almazor M, Bombardier C, Dougados M, Khanna D, King CM, Leong AL, Matteson EL, Schousboe JT, Moynihan E, Kolba KS, Jain A, Volkmann ER, Agrawal H, Bae S, Mudano AS, Patkar NM, Saag KG (2012) 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 64(5):625–639. https://doi.org/10.1002/acr.21641
Vassilopoulos D, Tsikrika S, Hatzara C, Podia V, Kandili A, Stamoulis N, Hadziyannis E (2011) Comparison of two gamma interferon release assays and tuberculin skin testing for tuberculosis screening in a cohort of patients with rheumatic diseases starting anti-tumor necrosis factor therapy. Clin Vaccine Immunol 18(12):2102–2108. https://doi.org/10.1128/CVI.05299-11
Gomez-Reino JJ, Carmona L, Angel Descalzo M et al (2007) Risk of tuberculosis in patients treated with tumor necrosis factor antagonists due to incomplete prevention of reactivation of latent infection. Arthritis Rheum 57(5):756–761. https://doi.org/10.1002/art.22768
Mariette X, Baron G, Tubach F, Lioté F, Combe B, Miceli-Richard C, Flipo RM, Goupille P, Allez M, Salmon D, Emilie D, Carcelain G, Ravaud P (2012) Influence of replacing tuberculin skin test with ex vivo interferon gamma release assays on decision to administer prophylactic antituberculosis antibiotics before anti-TNF therapy. Ann Rheum Dis 71(11):1783–1790. https://doi.org/10.1136/annrheumdis-2011-200408
Kilic L, Erden A, Sari A et al (2016) AB0350 rituximab seems outstanding biological option in late onset rheumatoid arthritis: Hur-Bio real life results: BMJ. Publishing Group Ltd, London
Armagan B, Sari A, Erden A et al (2018) Starting of biological disease modifying antirheumatic drugs may be postponed in rheumatoid arthritis patients with multimorbidity: single center real life results. Medicine 97(13):e9930. https://doi.org/10.1097/md.0000000000009930 [published Online First: 2018/03/30]
QuantiFERON®-TB Gold (QFT®) ELISA Package Insert. http://www.quantiferon.com/wp-content/uploads/2017/04/English_QFT_ELISA_R04_082016.pdf. Accessed August 2016
TC Sağlık Bakanlığı Halk Sağlığı Genel Müdürlüğü. Tüberküloz Tanı Rehberi (TC Ministry of Health Tuberculosis Diagnosis Guide), 2. Baskı. (2019). Artı6 Medya Tanıtım Matbaa Ltd. Şti. https://hsgm.saglik.gov.tr/depo/birimler/tuberkuloz_db/haberler/Tuberkuloz_Tani_Ve_Tedavi_Rehberi_/Tuberkuloz_Tani_ve_Tedavi_Rehberi.pdf. Ankara. Accessed May 2019
TC Sağlık Bakanlığı Anti-TNF Kullanan Hastalarda Tüberküloz Rehberi (TC Ministry of Health Tuberculosis Guide in Patients Using Anti-TNF) (2016). http://www.romatoloji.org/Dokumanlar/Site/ATKHTR.pdf. Ankara 2016
Tubach F, Salmon D, Ravaud P, Allanore Y, Goupille P, Bréban M, Pallot-Prades B, Pouplin S, Sacchi A, Chichemanian RM, Bretagne S, Emilie D, Lemann M, Lorthololary O, Mariette X, Research Axed on Tolerance of Biotherapies Group (2009) Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French research axed on tolerance of biotherapies registry. Arthritis Rheum 60(7):1884–1894. https://doi.org/10.1002/art.24632
Hsia EC, Schluger N, Cush JJ, Chaisson RE, Matteson EL, Xu S, Beutler A, Doyle MK, Hsu B, Rahman MU (2012) Interferon-gamma release assay versus tuberculin skin test prior to treatment with golimumab, a human anti-tumor necrosis factor antibody, in patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis. Arthritis Rheum 64(7):2068–2077. https://doi.org/10.1002/art.34382
Soborg B, Ruhwald M, Hetland ML et al (2009) Comparison of screening procedures for Mycobacterium tuberculosis infection among patients with inflammatory diseases. J Rheumatol 36(9):1876–1884. https://doi.org/10.3899/jrheum.081292
Martin J, Walsh C, Gibbs A, McDonnell T, Fearon U, Keane J, Codd MB, Dodd J, Veale D, FitzGerald O, Bresnihan B (2010) Comparison of interferon {gamma} release assays and conventional screening tests before tumour necrosis factor {alpha} blockade in patients with inflammatory arthritis. Ann Rheum Dis 69(1):181–185. https://doi.org/10.1136/ard.2008.101857
Smith R, Cattamanchi A, Steingart KR, Denkinger C, Dheda K, Winthrop KL, Pai M (2011) Interferon-gamma release assays for diagnosis of latent tuberculosis infection: evidence in immune-mediated inflammatory disorders. Curr Opin Rheumatol 23(4):377–384. https://doi.org/10.1097/BOR.0b013e3283474d62
TC Sağlık Bakanlığı (2015) Türkiye’de Verem Savaşı Raporu (TC Ministry of Health 2015 Tuberculosis Control Report in Turkey), Yayın No: 1059 (2016). Ankara. https://hsgm.saglik.gov.tr/depo/birimler/tuberkuloz_db/dosya/raporlar/Turkiyede_Verem_Savasi_2015_Raporu.pdf?type=file. Accessed 25 March 2017
WHO (2014) World Health Organization Global tuberculosis report. Geneva. Accessed 25 March 2017.
Lee H, Park HY, Jeon K, Jeong BH, Hwang JW, Lee J, Cha HS, Koh EM, Kang ES, Koh WJ (2015) QuantiFERON-TB Gold In-Tube assay for screening arthritis patients for latent tuberculosis infection before starting anti-tumor necrosis factor treatment. PLoS One 10(3):e0119260. https://doi.org/10.1371/journal.pone.0119260
Abubakar I, Drobniewski F, Southern J, Sitch AJ, Jackson C, Lipman M, Deeks JJ, Griffiths C, Bothamley G, Lynn W, Burgess H, Mann B, Imran A, Sridhar S, Tsou CY, Nikolayevskyy V, Rees-Roberts M, Whitworth H, Kon OM, Haldar P, Kunst H, Anderson S, Hayward A, Watson JM, Milburn H, Lalvani A, Adeboyeku D, Bari N, Barker J, Booth H, Chua F, Creer D, Darmalingam M, Davidson RN, Dedicoat M, Dunleavy A, Figueroa J, Haseldean M, Johnson N, Losewicz S, Lord J, Moore-Gillon J, Packe G, Pareek M, Tiberi S, Pozniak A, Sanderson F (2018) Prognostic value of interferon-γ release assays and tuberculin skin test in predicting the development of active tuberculosis (UK PREDICT TB): a prospective cohort study. Lancet Infect Dis 18(10):1077–1087
Matteelli A, Sulis G, Capone S, D’Ambrosio L, Migliori GB, Getahun H (2017) Tuberculosis elimination and the challenge of latent tuberculosis. Presse Med 46(2):e13–e21. https://doi.org/10.1016/j.lpm.2017.01.015
Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, Metcalfe JZ, Cattamanchi A, Dowdy DW, Dheda K, Banaei N (2014) Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 27(1):3–20. https://doi.org/10.1128/CMR.00034-13
Pai M, Zwerling A, Menzies D (2008) Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 149(3):177–184
Cagatay T, Aydin M, Sunmez S et al (2010) Follow-up results of 702 patients receiving tumor necrosis factor-alpha antagonists and evaluation of risk of tuberculosis. Rheumatol Int 30(11):1459–1463. https://doi.org/10.1007/s00296-009-1170-6
Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, Peloquin CA, Gordin FM, Nunes D, Strader DB, Bernardo J, Venkataramanan R, Sterling TR, ATS (American Thoracic Society) Hepatotoxicity of Antituberculosis Therapy Subcommittee (2006) An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med 174(8):935–952. https://doi.org/10.1164/rccm.200510-1666ST
Bray MG, Poulain C, Dougados M, Gossec L (2010) Frequency and tolerance of antituberculosis treatment according to national guidelines for prevention of risk of tuberculosis due to tumor necrosis factor blocker treatment. Joint Bone Spine 77(2):135–141. https://doi.org/10.1016/j.jbspin.2009.10.012
Bourre-Tessier J, Arino-Torregrosa M, Choquette D (2014) Increased incidence of liver enzymes abnormalities in patients treated with isoniazid in combination with disease modifying and/or biologic agents. Clin Rheumatol 33(8):1049–1053. https://doi.org/10.1007/s10067-014-2528-z
Hayashi PH, Fontana RJ, Chalasani NP et al (2015) Under-reporting and poor adherence to monitoring guidelines for severe cases of isoniazid hepatotoxicity. Clin Gastroenterol Hepatol 13(9):1676–1682.e1
LoBue PA, Moser KS (2003) Use of isoniazid for latent tuberculosis infection in a public health clinic. Am J Respir Crit Care Med 168(4):443–447
Control CfD, Prevention (2010) Severe isoniazid-associated liver injuries among persons being treated for latent tuberculosis infection-United States, 2004–2008. MMWR Morb Mortal Wkly Rep 59(8):224
Chalasani N, Bonkovsky HL, Fontana R et al (2015) Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterol 148(7):1340–1352.e7
Acknowledgments
We want to thank our research nurses M. Baykal and S. Ak.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Seyhoglu, E., Uyaroğlu, O.A., Erden, A. et al. QuantiFERON®-TB Gold In-Tube test can be used for screening latent tuberculosis before biological treatment in a Bacille Calmette-Guérin (BCG)-vaccinated country: the HUR-BIO single-center real-life results. Clin Rheumatol 40, 2027–2035 (2021). https://doi.org/10.1007/s10067-020-05443-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05443-3