Abstract
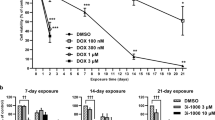
Doxorubicin (DOX) is an anthracycline antibiotic that exhibits high heart toxicity. Human-induced pluripotent stem cell-derived ventricular cardiomyocytes (hiPSC-vCMs) are important in vitro models for testing drug cardiotoxicity. Photobiomodulation therapy (PBMT) is a non-invasive therapy that stimulates cells growth and self-repair using light irradiation. This study aimed to investigate the in vitro effects of PBMT preconditioning on cardiotoxicity induced by DOX. HiPSC-vCMs were treated with PBMT for 500 s, followed by the addition of 2 μM DOX. LED irradiation preconditioning parameters were at 660 nm with an irradiance of 10 mW/cm2, performing 5 J/cm2, followed by 24-h DOX exposure (2 μM). Human iPSC-vCMs treated with 2 μM DOX or irradiated with PBMT composed the second and third groups, respectively. The control group did neither receive PBMT preconditioning nor DOX and was irradiated with a white standard lamp. Cells from all groups were collected to perform mRNA and miRNA expressions quantification. PBMT, when applied before the DOX challenge, restored the viability of hiPSC-vCMs and reduced ROS levels. Although downregulated by DOX, myocardial UCP2 mRNA expression presented marked upregulation after PBMT preconditioning. Expression of eNOS and UCP2 mRNA and NO production were decreased after DOX exposure, and PBMT preconditioning before the DOX challenge reversed these changes. Moreover, our data indicated that PBMT preconditioning lowered the miR-24 expression. Our data suggested that PBMT preconditioning ameliorated in vitro DOX-induced cardiotoxicity on transcription level, restoring NO levels and reducing oxidative stress.





Similar content being viewed by others
Data availability
All materials are available upon request to the corresponding author.
Code availability
Not applicable.
References
Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS et al (2009) Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem 16(25):3267–3285. https://doi.org/10.2174/092986709788803312
Songbo M, Lang H, Xinyong C, Bin X, Ping Z, Liang S (2019) Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol Lett 307:41–48. https://doi.org/10.1016/j.toxlet.2019.02.013
Li DL, Hill JA (2014) Cardiomyocyte autophagy and cancer chemotherapy. J Mol Cell Cardiol 71:54–61. https://doi.org/10.1016/j.yjmcc.2013.11.007
Chen RC, Xu XD, Zhi Liu X, Sun GB, Zhu YD, Dong X et al (2015) Total Flavonoids from Clinopodium chinense (Benth.) O. Ktze protect against doxorubicin-induced cardiotoxicity in vitro and in vivo. Evid Based Complement Alternat Med 2015:472565. https://doi.org/10.1155/2015/472565
Lipshultz SE, Alvarez JA, Scully RE (2008) Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart 94(4):525–533. https://doi.org/10.1136/hrt.2007.136093
Oliveira PJ, Bjork JA, Santos MS, Leino RL, Froberg MK, Moreno AJ et al (2004) Carvedilol-mediated antioxidant protection against doxorubicin-induced cardiac mitochondrial toxicity. Toxicol Appl Pharmacol 200(2):159–168. https://doi.org/10.1016/j.taap.2004.04.005
Rochette L, Guenancia C, Gudjoncik A, Hachet O, Zeller M, Cottin Y et al (2015) Anthracyclines/trastuzumab: new aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol Sci 36(6):326–348. https://doi.org/10.1016/j.tips.2015.03.005
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676. https://doi.org/10.1016/j.cell.2006.07.024
Da Rocha AM, Campbell K, Mironov S, Jiang J, Mundada L, Guerrero-Serna G et al (2017) hiPSC-CM monolayer maturation state determines drug responsiveness in high throughput pro-arrhythmia screen. Sci Rep 7(1):13834. https://doi.org/10.1038/s41598-017-13590-y
Eder A, Vollert I, Hansen A, Eschenhagen T (2016) Human engineered heart tissue as a model system for drug testing. Adv Drug Deliv Rev 96:214–224. https://doi.org/10.1016/j.addr.2015.05.010
De Rosa S, Curcio A, Indolfi C (2014) Emerging role of microRNAs in cardiovascular diseases. Circ J 78(3):567–575. https://doi.org/10.1253/circj.cj-14-0086
Santana ET, Feliciano RS, Serra AJ, Brigidio E, Antonio EL, Tucci PJF et al (2016) Comparative mRNA and MicroRNA profiling during acute myocardial infarction induced by coronary occlusion and ablation radio-frequency currents. Front Physiol 7:1–14. https://doi.org/10.3389/fphys.2016.00565
De Melo BL, Vieira SS, Antônio EL, dos Santos LFN, Portes LA, Feliciano RS et al (2016) Exercise training attenuates right ventricular remodeling in rats with pulmonary arterial stenosis. Front Physiol 7:541. https://doi.org/10.3389/fphys.2016.00541
Zhao L, Tao X, Qi Y, Xu L, Yin L, Peng J (2018) Protective effect of dioscin against doxorubicin-induced cardiotoxicity via adjusting microRNA-140-5p-mediated myocardial oxidative stress. Redox Biol 16:189–198. https://doi.org/10.1016/j.redox.2018.02.026
Lai L, Chen J, Wang N, Zhu G, Duan X, Ling F (2017) MiRNA-30e mediated cardioprotection of ACE2 in rats with doxorubicin-induced heart failure through inhibiting cardiomyocytes autophagy. Life Sci 169:69–75. https://doi.org/10.1016/j.lfs.2016.09.006
Gupta SK, Garg A, Avramopoulos P, Engelhardt S, Streckfuss-Bömeke K, Batkai S et al (2019) miR-212/132 Cluster modulation prevents doxorubicin-Mediated Atrophy and Cardiotoxicity. Mol Ther 27(1):17–28. https://doi.org/10.1016/j.ymthe.2018.11.004
Bortone F, Santos HA, Albertini R, Pesquero JB, Costa MS, Silva JA Jr (2008) Low level laser therapy modulates kinin receptors mRNA expression in the subplantar muscle of rat paw subjected to carrageenan-induced inflammation. Int Immunopharmacol 8(2):206–210. https://doi.org/10.1016/j.intimp.2007.09.004
Hamblin MR (2017) Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys 4(3):337–361. https://doi.org/10.3934/biophy.2017.3.337
Manchini MT, Serra AJ, Feliciano RS, Santana ET, Antônio EL, de Carvalho PTC et al (2014) Amelioration of cardiac function and activation of anti-inflammatory vasoactive peptides expression in the rat myocardium by low level laser therapy. PLoS ONE 9(7):e101270. https://doi.org/10.1371/journal.pone.0101270
Agrawal T, Gupta GK, Rai V, Carroll JD, Hamblin MR (2014) Preconditioning with low-level laser (light) therapy: light before the storm. Dose Response 12(4):619–649. https://doi.org/10.2203/dose-response.14-032.Agrawal
Liu Y, Zhang H (2016) Low-level laser irradiation precondition for cardiac regenerative therapy. Photomed Laser Surg 34(11):572–579. https://doi.org/10.1089/pho.2015.4058
Tuby H, Maltz L, Oron U (2006) Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis. Lasers Surg Med 38(7):682–688
Zhang H, Hou JF, Shen Y, Wang W, Wei YJ, Hu S (2010) Low level laser irradiation precondition to create friendly milieu of infarcted myocardium and enhance early survival of transplanted bone marrow cells. J Cell Mol Med 14(7):1975–1987. https://doi.org/10.1111/j.1582-4934.2009.00886.x
Zhu R, Blazeski A, Poon E, Costa KD, Tung L, Boheler KR (2014) Physical developmental cues for the maturation of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res Ther 5:117. https://doi.org/10.1186/scrt507
Mathur A, Loskill P, Shao K, Huebsch N, Hong S, Marcus SG et al (2015) Human iPSC-based cardiac microphysiological system for drug screening applications. Sci Rep 5:8883. https://doi.org/10.1038/srep08883
David R, Franz WM (2012) From pluripotency to distinct cardiomyocyte subtypes. Physiology 27:119–129. https://doi.org/10.1152/physiol.00044.2011
Xia N, Bollinger L, Steinkamp-Fenske K, Förstermann U, Li H (2010) Prunella vulgaris L Upregulates eNOS expression in human endothelial cells. Am J Chin Med 38(3):599–611. https://doi.org/10.1142/S0192415X10008081
Ankri R, Friedman H, Savion N, Kotev-Emeth S, Breitbart H, Lubart R (2010) Visible light induces nitric oxide (NO) formation in sperm and endothelial cells. Lasers Surg Med 42(4):348–352. https://doi.org/10.1002/lsm.20849
Rizzi M, Migliario M, Tonello S, Rocchetti V, Renò F (2018) Photobiomodulation induces in vitro re-epithelialization via nitric oxide production. Lasers Med Sci 33(5):1003–1008. https://doi.org/10.1007/s10103-018-2443-7
Huang SJ, Lee SY, Teng YH, Lee SD, Cheng YJ (2021) Photobiomodulation therapy to promote angiogenesis in diabetic mice with hindlimb ischemia. Photobiomodul Photomed Laser Surg 39(7):453–462. https://doi.org/10.1089/photob.2020.4896
Zhang R, Mio Y, Pratt PF, Lohr N, Warltier DC, Whelan HT et al (2009) Near infrared light protects cardiomyocytes from hypoxia and reoxygenation injury by a nitric oxide dependent mechanism. J Mol Cell Cardiol 46(1):4–14. https://doi.org/10.1016/j.yjmcc.2008.09.707
Oishi JC, De Moraes TF, Buzinari TC, Cárnio EC, Parizotto NA, Rodrigues GJ (2017) Hypotensive acute effect of photobiomodulation therapy on hypertensive rats. Life Sci 178:56–60. https://doi.org/10.1016/j.lfs.2017.04.011
Buzinari TC, de Moraes TF, Cárnio EC, Lopes LA, Salgado HC, Rodrigues GJ (2020) Photobiomodulation induces hypotensive effect in spontaneously hypertensive rats. Lasers Med Sci 35(3):567–572. https://doi.org/10.1007/s10103-019-02849-7
Totzeck M, Hendgen-Cotta UB, Rassaf T (2017) Nitrite-nitric oxide signaling and cardioprotection. Adv Exp Med Biol 982:335–346. https://doi.org/10.1007/978-3-319-55330-6_18
Rassaf T, Preik M, Kleinbongard P, Lauer T, Hei C, Strauer BE et al (2002) Evidence for in vivo transport of bioactive nitric oxide in human plasma. J Clin Invest 109(9):1241–1248. https://doi.org/10.1172/JCI14995
Mu H, Liu H, Zhang J, Huang J, Zhu C, Lu Y, Shi Y, Wang Y (2019) Ursolic acid prevents doxorubicin-induced cardiac toxicity in mice through eNOS activation and inhibition of eNOS uncoupling. J Cell Mol Med 23(3):2174–2183. https://doi.org/10.1111/jcmm.14130
Dutra MRH, Feliciano RS, Jacinto KR, Gouveia TLF, Brigidio E, Serra AJ et al (2018) Protective role of UCP2 in oxidative stress and apoptosis during the silent phase of an experimental model of epilepsy induced by pilocarpine. Oxi Med Cell Logev 2018:6736721. https://doi.org/10.1155/2018/6736721
Nègre-Salvayre A, Hirtz C, Carrera G, Cazenave R, Troly M, Salvayre R et al (1997) A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J 11(10):809–815
Kizaki T, Suzuki K, Hitomi Y, Taniguchi N, Saitoh D, Watanabe K, Onoé K, Day NK, Good RA, Ohno H (2002) Uncoupling protein 2 plays an important role in nitric oxide production of lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA 99(14):9392–7. https://doi.org/10.1073/pnas.142206299
Lee KU, Lee IK, Han J, Song DK, Kim YM, Song HS, Kim HS, Lee WJ, Koh EH, Song KH, Han SM, Kim MS, Park IS, Park JY (2005) Effects of recombinant adenovirus-mediated uncoupling protein 2 overexpression on endothelial function and apoptosis. Circ Res 96(11):1200–1207. https://doi.org/10.1161/01.RES.0000170075.73039.5b
Fallahi AA, Shekarfroush S, Rahimi M, Jalali A, Khoshbaten A (2016) Alteration in cardiac uncoupling proteins and eNOS gene expression following high-intensity interval training in favor of increasing mechanical efficiency. Iran J Basic Med Sci 19(3):258–264
Hong J, Park E, Lee J, Lee Y, Rooney BV, Park Y (2021) Exercise training mitigates ER stress and UCP2 deficiency-associated coronary vascular dysfunction in atherosclerosis. Sci Rep 11(1):15449. https://doi.org/10.1038/s41598-021-94944-5
Wang Y, Lei T, Yuan J, Wu Y, Shen X, Gao J et al (2018) GCN2 deficiency ameliorates doxorubicin-induced cardiotoxicity by decreasing cardiomyocyte apoptosis and myocardial oxidative stress. Redox Biol 17:25–34. https://doi.org/10.1016/j.redox.2018.04.009
Hu C, Zhang X, Wei W, Zhang N, Wu H, Ma Z, Li L, Deng W, Tang Q (2019) Matrine attenuates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via maintaining AMPKα/UCP2 pathway. Acta Pharm Sin B 9(4):690–701. https://doi.org/10.1016/j.apsb.2019.03.003
Li Q, Li C, Xi S, Li X, Ding L, Li M (2019) The effects of photobiomodulation therapy on mouse pre-osteoblast cell line MC3T3-E1 proliferation and apoptosis via miR-503/Wnt3a pathway. Lasers Med Sci 34(3):607–614. https://doi.org/10.1007/s10103-018-2636-0
Feliciano RS, Atum ALB, Ruiz EGS, Serra AJ, Antonio EL, Manchini MT, Silva JMAS, Tucci PJF, Nathanson L, Morris M, Chavantes MC, Silva JA Jr (2021) Photobiomodulation therapy on myocardial infarction in rats: transcriptional implications to cardiac remodeling. Lasers Surg Med. https://doi.org/10.1002/lsm.23407
Fiedler J, Jazbutyte V, Kirchmaier BC, Gupta SK, Lorenzen J, Hartmann D et al (2011) MicroRNA-24 regulates vascularity after myocardial infarction. Circulation 124(6):720–730. https://doi.org/10.1161/CIRCULATIONAHA.111.039008
Qian L, Van Laake LW, Huang Y, Liu S, Wendland MF, Srivastava D (2011) miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med 208(3):549–560. https://doi.org/10.1084/jem.20101547
Guo C, Deng Y, Liu J, Qian L (2015) Cardiomyocyte-specific role of miR-24 in promoting cell survival. J Cell Mol Med 19(1):103–112. https://doi.org/10.1111/jcmm.12393
Meloni M, Marchetti M, Garner K, Littlejohns B, Sala-Newby G, Xenophontos N et al (2013) Local inhibition of microRNA-24 improves reparative angiogenesis and left ventricle remodeling and function in mice with myocardial infarction. Mol Ther 21(7):1390–1402. https://doi.org/10.1038/mt.2013.89
Yang J, Xu J, Danniel M, Wang X, Wang W, Zeng L, Shen L (2018) The interaction between XBP1 and eNOS contributes to endothelial cell migration. Exp Cell Res 363(2):262–270. https://doi.org/10.1016/j.yexcr.2018.01.016
Wang M, Sun L, Ding W, Cai S, Zhao Q (2019) Ablation alleviates atrial fibrillation by regulating endothelial nitric oxide signaling pathways synthase/nitric oxide via miR-155-5p and miR-24-3p. J Cell Biochem 120(3):4451–4462. https://doi.org/10.1002/jcb.27733
Li HM, Mo ZW, Peng YM, Li Y, Dai WP, Yuan HY, Chang FJ, Wang TT, Wang M, Hu KH, Li XD, Ning DS, Chen YT, Song YK, Lu XL, Pei Z, Dong YG, Wang ZP, Zhang X, Xu YQ, Wang SM, Ou ZJ, Ou JS (2020) Angiogenic and antiangiogenic mechanisms of high-density lipoprotein from healthy subjects and coronary artery diseases patients. Redox Biol 36:101642. https://doi.org/10.1016/j.redox.2020.101642
Marchetti M, Meloni M, Anwar M, Zen AAH, Sala-Newby G, Slater S, Ford K, Caporali A, Emanueli C (2020) MicroRNA-24-3p Targets notch, and other vascular morphogens to regulate post-ischemic microvascular responses in limb muscles. Int J Mol Sci 21(5):1733. https://doi.org/10.3390/ijms21051733
Wang J, Huang W, Xu R, Nie Y, Cao X, Meng J et al (2012) MicroRNA-24 regulates cardiac fibrosis after myocardial infarction. J Cell Mol Med 16(9):2150–2160. https://doi.org/10.1111/j.1582-4934.2012.01523.x
Funding
The present work was supported by grant number 2009/54225–8 from the São Paulo Research Foundation (FAPESP) and National Council for Scientific and Technological (CNPq grant number 304607/2018–5).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Allan Luís Barboza Atum, José Almir Alves da Silva, and Danila Marques. Equipment and drugs were supplied by Renato Araújo Prates, Fernanda Marciano Consolim-Colombo, and Maria Claudia Costa Irigoyen. The first draft of the manuscript was written by Maria Cristina Chavantes and José Antônio Silva Júnior. Final version of the manuscript was written and edited by José Antônio Silva Júnior, and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Atum, A.L.B., da Silva, J.A.A., Marques, D. et al. Photobiomodulation therapy preconditioning modifies nitric oxide pathway and oxidative stress in human-induced pluripotent stem cell-derived ventricular cardiomyocytes treated with doxorubicin. Lasers Med Sci 37, 1667–1675 (2022). https://doi.org/10.1007/s10103-021-03416-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-021-03416-9