Abstract
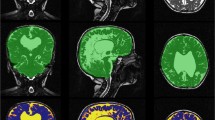
To assess automated volumetric analysis as a potential presurgical diagnostic tool or as a method to potentially shed light on normal pressure hydrocephalus (NPH) pathophysiology. MRI imaging according to our protocol was performed in 29 NPH patients, 45 non-NPH (but suspected) patients and 15 controls. Twenty patients underwent a second MRI 3 months after ventriculoperitoneal (VP) shunt surgery. All structures relevant to NPH diagnosis were automatically segmented using commercial software. The results were subsequently tested using ANOVA analysis. Significant differences in the volumes of the corpus callosum, left hippocampus, internal globus pallidus, grey and white matter and ventricular volumes were observed between NPH group and healthy controls. However, the differences between NPH and non-NPH groups were non-significant. Three months after, VP shunt insertion decreased ventricular volume was the only clearly significant result (p value 0.0001). Even though a detailed volumetric study shows several significant differences, volumetric analysis as a standalone method does not provide a simple diagnostic biomarker, nor does it shed a light on an unknown NPH aetiology.







Similar content being viewed by others
References
Adams RDFC, Hakim S (1965) Symptomatic occult hydrocephalus with “normal” cerebrospinal fluid pressure. A treatable syndrome. N Engl J Med 273:117–226
Allali G, Laidet M, Armand S, Saj A, Krack P, Assal F (2018) Apathy in idiopathic normal pressure hydrocephalus: a marker of reversible gait disorders. Int J Geriatr Psychiatry 33:735–742. https://doi.org/10.1002/gps.4847
Andrejevic M, Meshi D, van den Bos W, Heekeren HR (2017) Individual differences in social desirability are associated with white-matter microstructure of the external capsule. Cogn Affect Behav Neurosci 17:1255–1264. https://doi.org/10.3758/s13415-017-0548-2
Ashburner J (2012) SPM: a history. Neuroimage 62:791–800. https://doi.org/10.1016/j.neuroimage.2011.10.025
Bauer E, Toepper M, Gebhardt H, Gallhofer B, Sammer G (2015) The significance of caudate volume for age-related associative memory decline. Brain Res 1622:137–148. https://doi.org/10.1016/j.brainres.2015.06.026
Boon AJ, Tans JT, Delwel EJ, Egeler-Peerdeman SM, Hanlo PW, Wurzer JA, Hermans J (1997) Dutch normal pressure hydrocephalus study: baseline characteristics with emphasis on clinical findings. Eur J Neurol 4:39–47. https://doi.org/10.1111/j.1468-1331.1997.tb00297.x
Braak H, Braak E (1990) Alzheimer’s disease: striatal amyloid deposits and neurofibrillary changes. J Neuropathol Exp Neurol 49:215–224
Bradley WG Jr (2016) Magnetic resonance imaging of normal pressure hydrocephalus. Semin Ultrasound CT MR 37:120–128. https://doi.org/10.1053/j.sult.2016.01.005
de Jong LW, van der Hiele K, Veer IM, Houwing JJ, Westendorp RG, Bollen EL, de Bruin PW, Middelkoop HA, van Buchem MA, van der Grond J (2008) Strongly reduced volumes of putamen and thalamus in Alzheimer’s disease: an MRI study. Brain 131:3277–3285. https://doi.org/10.1093/brain/awn278
Devito EE, Pickard JD, Salmond CH, Iddon JL, Loveday C, Sahakian BJ (2005) The neuropsychology of normal pressure hydrocephalus (NPH). Br J Neurosurg 19:217–224. https://doi.org/10.1080/02688690500201838
Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, Deichmann R, Ashburner J, Frackowiak RS (2008) Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci 28:7143–7152. https://doi.org/10.1523/JNEUROSCI.1486-08.2008
El Ahmadieh TY, Wu EM, Kafka B, Caruso JP, Neeley OJ, Plitt A, Aoun SG, Olson DM, Ruchinskas RA, Cullum CM, Barnett S, Welch BG, Batjer HH, White JA (2019) Lumbar drain trial outcomes of normal pressure hydrocephalus: a single-center experience of 254 patients. J Neurosurg:1–7. https://doi.org/10.3171/2018.8.JNS181059
Feletti A, d'Avella D, Wikkelso C, Klinge P, Hellstrom P, Tans J, Kiefer M, Meier U, Lemcke J, Paterno V, Stieglitz L, Sames M, Saur K, Kordas M, Vitanovic D, Gabarros A, Llarga F, Triffaux M, Tyberghien A, Juhler M, Hasselbalch S, Cesarini K, Laurell K (2019) Ventriculoperitoneal shunt complications in the European idiopathic normal pressure hydrocephalus multicenter study. Oper Neurosurg (Hagerstown) 17:97–102. https://doi.org/10.1093/ons/opy232
Ghosh S, Lippa C (2014) Diagnosis and prognosis in idiopathic normal pressure hydrocephalus. Am J Alzheimer’s Dis Other Dement 29:583–589. https://doi.org/10.1177/1533317514523485
Goto M, Abe O, Aoki S, Kamagata K, Hori M, Miyati T, Gomi T, Takeda T (2018) Combining segmented grey and white matter images improves voxel-based morphometry for the case of dilated lateral ventricles. Magn Reson Med Sci 17:293–300. https://doi.org/10.2463/mrms.mp.2017-0127
Grahn JA, Parkinson JA, Owen AM (2008) The cognitive functions of the caudate nucleus. Prog Neurobiol 86:141–155. https://doi.org/10.1016/j.pneurobio.2008.09.004
Greitz D (2004) Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg Rev 27:145–165; discussion 166-147. https://doi.org/10.1007/s10143-004-0326-9
Iddon JL, Pickard JD, Cross JJL, Griffiths PD, Czosnyka M, Sahakian BJ (1999) Specific patterns of cognitive impairment in patients with idiopathic normal pressure hydrocephalus and Alzheimer’s disease: a pilot study. J Neurol Neurosurg Psychiatry 67:723–732. https://doi.org/10.1136/jnnp.67.6.723
Jaraj D, Rabiei K, Marlow T, Jensen C, Skoog I, Wikkelso C (2014) Prevalence of idiopathic normal-pressure hydrocephalus. Neurology 82:1449–1454. https://doi.org/10.1212/WNL.0000000000000342
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) Fsl. Neuroimage 62:782–790. https://doi.org/10.1016/j.neuroimage.2011.09.015
Katzman R, Hussey F (1970) A simple constant-infusion manometric test for measurement of CSF absorption. I Rationale and method. Neurology 20:534–544. https://doi.org/10.1212/wnl.20.6.534
Keong NC, Pena A, Price SJ, Czosnyka M, Czosnyka Z, Pickard JD (2016) Imaging normal pressure hydrocephalus: theories, techniques, and challenges. Neurosurg Focus 41:E11. https://doi.org/10.3171/2016.7.FOCUS16194
Kim MJ, Seo SW, Lee KM, Kim ST, Lee JI, Nam DH, Na DL (2011) Differential diagnosis of idiopathic normal pressure hydrocephalus from other dementias using diffusion tensor imaging. AJNR Am J Neuroradiol 32:1496–1503. https://doi.org/10.3174/ajnr.A2531
Kockum K, Virhammar J, Riklund K, Soderstrom L, Larsson EM, Laurell K (2019) Standardized image evaluation in patients with idiopathic normal pressure hydrocephalus: consistency and reproducibility. Neuroradiology. 61:1397–1406. https://doi.org/10.1007/s00234-019-02273-2
Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM (2005) The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 57:S17–S28; discussion ii-v. https://doi.org/10.1227/01.neu.0000168184.01002.60
Marmarou A, Sawauchi S, Dunbar J (2005) Diagnosis and management of idiopathic normal-pressure hydrocephalus: a prospective study in 151 patients. J Neurosurg 102:11
Moore DW, Kovanlikaya I, Heier LA, Raj A, Huang C, Chu KW, Relkin NR (2012) A pilot study of quantitative MRI measurements of ventricular volume and cortical atrophy for the differential diagnosis of normal pressure hydrocephalus. Neurol Res Int 2012:718150. https://doi.org/10.1155/2012/718150
Motl RW, Hubbard EA, Sreekumar N, Wetter NC, Sutton BP, Pilutti LA, Sosnoff JJ, Benedict RH (2015) Pallidal and caudate volumes correlate with walking function in multiple sclerosis. J Neurol Sci 354:33–36. https://doi.org/10.1016/j.jns.2015.04.041
Ogino A, Kazui H, Miyoshi N, Hashimoto M, Ohkawa S, Tokunaga H, Ikejiri Y, Takeda M (2006) Cognitive impairment in patients with idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord 21:113–119. https://doi.org/10.1159/000090510
Palm WM, Walchenbach R, Bruinsma B, Admiraal-Behloul F, Middelkoop HA, Launer LJ, van der Grond J, van Buchem MA (2006) Intracranial compartment volumes in normal pressure hydrocephalus: volumetric assessment versus outcome. AJNR Am J Neuroradiol 27:76–79
Peterson KA, Mole TB, Keong NCH, DeVito EE, Savulich G, Pickard JD, Sahakian BJ (2018) Structural correlates of cognitive impairment in normal pressure hydrocephalus. Acta Neurol Scand. https://doi.org/10.1111/ane.13052
Poulin SP, Dautoff R, Morris JC, Barrett LF, Dickerson BC, Alzheimer’s Disease Neuroimaging I (2011) Amygdala atrophy is prominent in early Alzheimer’s disease and relates to symptom severity. Psychiatry Res 194:7–13. doi:https://doi.org/10.1016/j.pscychresns.2011.06.014
Ravdin LD, Katzen HL, Jackson AE, Tsakanikas D, Assuras S, Relkin NR (2008) Features of gait most responsive to tap test in normal pressure hydrocephalus. Clin Neurol Neurosurg 110:455–461. https://doi.org/10.1016/j.clineuro.2008.02.003
Ruitenberg A, den Heijer T, Bakker SLM, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MMB (2005) Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Ann Neurol 57:789–794. https://doi.org/10.1002/ana.20493
Sakakibara R, Kanda T, Sekido T, Uchiyama T, Awa Y, Ito T, Liu Z, Yamamoto T, Yamanishi T, Yuasa T, Shirai K, Hattori T (2008) Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourol Urodyn 27:507–510. https://doi.org/10.1002/nau.20547
Sakhare AR, Barisano G, Pa J (2019) Assessing test-retest reliability of phase contrast MRI for measuring cerebrospinal fluid and cerebral blood flow dynamics. Magn Reson Med 82:658–670. https://doi.org/10.1002/mrm.27752
Savolainen S, Laakso MP, Paljarvi L, Alafuzoff I, Hurskainen H, Partanen K, Soininen H, Vapalahti M (2000) MR imaging of the hippocampus in normal pressure hydrocephalus: correlations with cortical Alzheimer’s disease confirmed by pathologic analysis. AJNR Am J Neuroradiol 21:409–414
Shinoda N, Hirai O, Hori S, Mikami K, Bando T, Shimo D, Kuroyama T, Kuramoto Y, Matsumoto M, Ueno Y (2017) Utility of MRI-based disproportionately enlarged subarachnoid space hydrocephalus scoring for predicting prognosis after surgery for idiopathic normal pressure hydrocephalus: clinical research. J Neurosurg 127:1436–1442. https://doi.org/10.3171/2016.9.JNS161080
Siasios I, Kapsalaki EZ, Fountas KN, Fotiadou A, Dorsch A, Vakharia K, Pollina J, Dimopoulos V (2016) The role of diffusion tensor imaging and fractional anisotropy in the evaluation of patients with idiopathic normal pressure hydrocephalus: a literature review. Neurosurg Focus 41:E12. https://doi.org/10.3171/2016.6.FOCUS16192
Skalický P, Mládek A, Vlasák A, De Lacy P, Beneš V, Bradáč O (2019) Normal pressure hydrocephalus—an overview of pathophysiological mechanisms and diagnostic procedures. Neurosurg Rev:1–14. https://doi.org/10.1007/s10143-019-01201-5
Spiegelhalder K, Regen W, Prem M, Baglioni C, Nissen C, Feige B, Schnell S, Kiselev VG, Hennig J, Riemann D (2014) Reduced anterior internal capsule white matter integrity in primary insomnia. Hum Brain Mapp 35:3431–3438
Swartz RH, Black SE (2006) Anterior-medial thalamic lesions in dementia: frequent, and volume dependently associated with sudden cognitive decline. J Neurol Neurosurg Psychiatry 77:1307–1312. https://doi.org/10.1136/jnnp.2006.091561
Tekin S, Cummings JL (2002) Frontal–subcortical neuronal circuits and clinical neuropsychiatry an update. J Psychosom Res 8
Tsakanikas D, Relkin N (2007) Normal pressure hydrocephalus. Semin Neurol 27:058–065. https://doi.org/10.1055/s-2006-956756
Tullberg M, Persson J, Petersen J, Hellstrom P, Wikkelso C, Lundgren-Nilsson A (2018) Shunt surgery in idiopathic normal pressure hydrocephalus is cost-effective-a cost utility analysis. Acta Neurochir 160:509–518. https://doi.org/10.1007/s00701-017-3394-7
van Harten B, Courant MNJ, Scheltens P, Weinstein HC (2004) Validation of the HIV dementia scale in an elderly cohort of patients with subcortical cognitive impairment caused by subcortical ischaemic vascular disease or a normal pressure hydrocephalus. Dement Geriatr Cogn Disord 18:109–114. doi:https://doi.org/10.1159/000077818
Walchenbach R, Geiger E, Thomeer RTWM, Vanneste JAL (2002) The value of temporary external lumbar CSF drainage in predicting the outcome of shunting on normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 72:503–506. https://doi.org/10.1136/jnnp.72.4.503
Wang XD, Ren M, Zhu MW, Gao WP, Zhang J, Shen H, Lin ZG, Feng HL, Zhao CJ, Gao K (2015) Corpus callosum atrophy associated with the degree of cognitive decline in patients with Alzheimer’s dementia or mild cognitive impairment: a meta-analysis of the region of interest structural imaging studies. J Psychiatr Res 63:10–19. https://doi.org/10.1016/j.jpsychires.2015.02.005
Zare A, Jahanshahi A, Rahnama'i MS, Schipper S, van Koeveringe GA (2019) The role of the periaqueductal gray matter in lower urinary tract function. Mol Neurobiol 56:920–934. https://doi.org/10.1007/s12035-018-1131-8
Zhang H, Reitz A, Kollias S, Summers P, Curt A, Schurch B (2005) An fMRI study of the role of suprapontine brain structures in the voluntary voiding control induced by pelvic floor contraction. NeuroImage 24:174–180. https://doi.org/10.1016/j.neuroimage.2004.08.027
Ziegelitz D, Arvidsson J, Hellström P, Tullberg M, Wikkelsø C, Starck G (2016) Pre-and postoperative cerebral blood flow changes in patients with idiopathic normal pressure hydrocephalus measured by computed tomography (CT)-perfusion. J Cereb Blood Flow Metab 36:1755–1766. https://doi.org/10.1177/0271678X15608521
Funding
Supported by institutional grant Czech Ministry of Defence MO1012.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Military University Hospital Ethical committee.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vlasák, A., Skalický, P., Mládek, A. et al. Structural volumetry in NPH diagnostics and treatment—future or dead end?. Neurosurg Rev 44, 503–514 (2021). https://doi.org/10.1007/s10143-020-01245-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01245-y