Abstract
Background
Compared to other esophageal cancers, clinical stage IA esophageal cancer generally has a good prognosis, although a subgroup of patients has a poor prognosis. Unfortunately, clinical diagnoses of invasion depth or lymph node metastasis are not always accurate, which make it difficult to identify patients with a high risk of postoperative recurrence using the tumor-node-metastasis staging system. Fluorodeoxyglucose-positron emission tomography may help guide the identification of malignant tumors and the evaluation of their malignant grade based on glucose metabolism. We aimed to evaluate the association between pre-operative fluorodeoxyglucose-positron emission tomography findings and the postoperative prognosis of patients with clinical stage IA esophageal cancer.
Methods
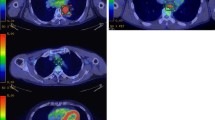
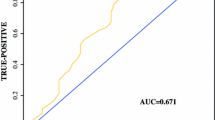
This single-center retrospective study evaluated pre-esophagectomy fluorodeoxyglucose-positron emission tomography findings from 38 patients with clinical stage IA esophageal cancer. Receiver operating characteristic curve analysis was performed to evaluate the prognostic significance of the primary tumor having low and high SUVmax values (cut-off: 3.56).
Results
Overall survival (log-rank p = 0.034) and progression-free survival (log-rank p = 0.008) were significantly different between the groups with low SUVmax values (n = 18) and high SUVmax values (n = 20). Furthermore, the primary tumor’s SUVmax value was related to pathological vascular invasion (p = 0.045) and distant metastasis (p = 0.042).
Conclusion
The SUVmax of the primary tumor is a predictor of postoperative survival for clinical stage IA esophageal cancer. Thus, using fluorodeoxyglucose-positron emission tomography to evaluate the primary tumor’s glucose metabolism may reflect the tumor’s grade and potentially compensate for inaccuracies in tumor-node-metastasis staging.



Similar content being viewed by others
References
Kitagawa Y, Uno T, Oyama T et al (2019) Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal society: part 1. Esophagus 16:1–24
Endo M, Kawano T (1997) Detection and classification of early squamous cell esophageal cancer. Dis Esophagus 10:155–158
Muto M, Minashi K, Yano T et al (2010) Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol 28:1566–1572
Ishihara R, Iishi H, Uedo N et al (2008) Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 68:1066–1072
Katada C, Tanabe S, Wada T et al (2019) Retrospective assessment of the diagnostic accuracy of the depth of invasion by narrow band imaging magnifying endoscopy in patients with superficial esophageal squamous cell carcinoma. J Gastrointest Cancer 50:292–297
Mizumoto T, Hiyama T, Oka S et al (2018) Diagnosis of superficial esophageal squamous cell carcinoma invasion depth before endoscopic submucosal dissection. Dis Esophagus. https://doi.org/10.1093/dote/dox142
McGuill MJ, Byrne P, Ravi N et al (2008) The prognostic impact of occult lymph node metastasis in cancer of the esophagus or esophago-gastric junction: systematic review and meta-analysis. Dis Esophagus 21:236–240
Aoyama J, Kawakubo H, Mayanagi S et al (2019) Discrepancy between the clinical and final pathological findings of lymph node metastasis in superficial esophageal cancer. Ann Surg Oncol 26:2874–2881
Rigo P, Paulus P, Kaschten BJ et al (1996) Oncological applications of positron emission tomography with fluorine-18 fluorodeoxyglucose. Eur J Nucl Med 23:1641–1674
Kato H, Kuwano H, Nakajima M et al (2002) Comparison between positron emission tomography and computed tomography in the use of the assessment of esophageal carcinoma. Cancer 94:921–928
Guo H, Zhu H, Xi Y et al (2007) Diagnostic and prognostic value of 18F-FDG PET/CT for patients with suspected recurrence from squamous cell carcinoma of the esophagus. J Nucl Med 48:1251–1258
Brücher BL, Weber W, Bauer M et al (2001) Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Ann Surg 233:300–309
Downey RJ, Akhrust T, Ilson D et al (2003) Whole body 18FDGPET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 21:428–432
Miyata H, Yamasaki M, Takahashi T et al (2014) Determinants of response to neoadjuvant chemotherapy for esophageal cancer using 18F-fluorodeoxiglucose positron emission tomography (18F-FDG-PET). Ann Surg Oncol 21:575–582
Miyata H, Doki Y, Yasuda T et al (2008) Evaluation of clinical significance of 18F-fluorodeoxyglucose positron emission tomography in superficial squamous cell carcinomas of the thoracic esophagus. Dis Esophagus 21:144–150
Kita Y, Okumura H, Uchikado Y et al (2013) Clinical significance of 18F-fluorodeoxyglucose positron emission tomography in superficial esophageal squamous cell carcinoma. Ann Surg Oncol 20:1646–1652
Nakajima M, Muroi H, Yokoyama H et al (2018) 18 F-Fluorodeoxyglucose positron emission tomography can be used to determine the indication for endoscopic resection of superficial esophageal cancer. Cancer Med 7:3604–3610
Rice TW, Gress DM, Patil DT et al (2017) Cancer of the esophagus and esophagogastric junction-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67:304–317
Japan Esophageal Society (2017) Japanese Classification of Esophageal Cancer, 11th edition: part I. Esophagus 14:1–36
Brown C, Howes B, Jamieson GG et al (2012) Accuracy of PET-CT in predicting survival in patients with esophageal cancer. World J Surg 36:1089–1095
Yasuda T, Yano M, Miyata H et al (2015) Prognostic significance of (18)F-fluorodeoxyglucose positron emission tomography (FDG-PET)-positive lymph nodes following neoadjuvant chemotherapy and surgery for resectable thoracic esophageal squamous cell carcinoma. Ann Surg Oncol 22:2599–2607
Hamai Y, Hihara J, Emi M et al (2016) Ability of fluorine-18 fluorodeoxyglucose positron emission tomography to predict outcomes of neoadjuvant chemoradiotherapy followed by surgical treatment for esophageal squamous cell carcinoma. Ann Thorac Surg 102:1132–1139
Izumi D, Yoshida N, Watanabe M et al (2016) Tumor/normal esophagus ratio in (18)F-fluorodeoxyglucose positron emission tomography/computed tomography for response and prognosis stratification after neoadjuvant chemotherapy for esophageal squamous cell carcinoma. J Gastroenterol 51:788–795
Furukawa T, Hamai Y, Hihara J et al (2016) Clinical significance of FDG-PET to predict pathologic tumor invasion and lymph node metastasis of superficial esophageal squamous cell carcinoma. Ann Surg Oncol 23:4086–4092
Nakajima Y, Nagai K, Miyake S et al (2002) Evaluation of an indicator for lymph node metastasis of esophageal squamous cell carcinoma invading the submucosal layer. Jpn J Cancer Res. 93(3):305–312
Shimada H, Nabeya Y, Matsubara H et al (2006) Prediction of lymph node status in patients with superficial esophageal carcinoma: analysis of 160 surgically resected cancers. Am J Surg. 191(2):250–254
Stein HJ, Feith M, Bruecher BL et al (20015) Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg 242(4):566–573 (discussion 573–5)
Altorki NK, Lee PC, Liss Y et al (2008) Multifocal neoplasia and nodal metastases in T1 esophageal carcinoma: implications for endoscopic treatment. Ann Surg. 247(3):434–439. https://doi.org/10.1097/SLA.0b013e318163a2ff
Ancona E, Rampado S, Cassaro M et al (2008) Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol. 15(11):3278–3288. https://doi.org/10.1245/s10434-008-0065-1(Epub 2008 Aug 26)
Tomita N, Matsumoto T, Hayashi T et al (2008) Lymphatic invasion according to D2–40 immunostaining is a strong predictor of nodal metastasis in superficial squamous cell carcinoma of the esophagus: algorithm for risk of nodal metastasis based on lymphatic invasion. Pathol Int. 58(5):282–287. https://doi.org/10.1111/j.1440-1827.2008.02224.x
Tachibana M, Hirahara N, Kinugasa S et al (2008) Clinicopathologic features of superficial esophageal cancer: results of consecutive 100 patients. Ann Surg Oncol. 15(1):104–116 (Epub 2007 Sep 22)
Chiba T, Kawachi H, Kawano T et al (2010) Independent histological risk factors for lymph node metastasis of superficial esophageal squamous cell carcinoma; implication of claudin-5 immunohistochemistry for expanding the indications of endoscopic resection. Dis Esophagus. 23(5):398–407. https://doi.org/10.1111/j.1442-2050.2009.01023.x(Epub 2009 Nov 9)
Zhuge L, Wang S, Xie J et al (2018) A model based on endoscopic morphology of submucosal esophageal squamous cell carcinoma for determining risk of metastasis on lymph nodes. J Thorac Dis. 10(12):6846–6853. https://doi.org/10.21037/jtd.2018.11.77
Kato H, Miyazaki T, Nakajima M et al (2005) The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer 103(1):148–156
Akutsu Y, Kato K, Igaki H et al (2016) The prevalence of overall and initial lymph node metastases in clinical T1N0 thoracic esophageal cancer: from the results of JCOG0502, a prospective multicenter study. Ann Surg 264:1009–1015
Yamashina T, Ishihara R, Nagai K et al (2013) Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am J Gastroenterol 108:544–551
Kurokawa Y, Muto M, Minashi K et al (2009) A phase II trial of combined treatment of endoscopic mucosal resection and chemoradiotherapy for clinical stage I esophageal carcinoma: Japan Clinical Oncology Group Study JCOG0508. Jpn J Clin Oncol 39:686–689
Ando N, Kato H, Igaki H et al (2012) A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 19(1):68–74. https://doi.org/10.1245/s10434-011-2049-9(Epub 2011 Aug 31)
Jingu K, Umezawa R, Yamamoto T et al (2019) FDG-PET might not contribute to improving survival in patients with locally advanced inoperable esophageal cancer. Int J Clin Oncol 24:927–933
Makino T, Yamasaki M, Tanaka K et al (2019) (2018) Metabolic tumor volume change predicts long-term survival and histological response to preoperative chemotherapy in locally advanced esophageal cancer. Ann Surg 270(6):1090–1095. https://doi.org/10.1097/SLA.0000000000002808
Acknowledgements
The authors thank Dr. Yukihiro Watanabe (a biostatistician) for advice on statistical methods.
Author information
Authors and Affiliations
Contributions
YM: Study conception and design, collecting, analyzing, and interpreting the data, and writing the manuscript. HS: Study conception and design. NF: Collecting the data. SO: Collecting the data. HS: Collecting the data. YH: Collecting the data. TY: Analyzing and interpreting the data. SS: Critical intellectual contributions. KO: Critical intellectual contributions. SY: Critical intellectual contributions. IK: Study supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Formal consent was not required for this type of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Miyawaki, Y., Sato, H., Fujiwara, N. et al. Association of the primary tumor’s SUVmax with survival after surgery for clinical stage IA esophageal cancer: a single-center retrospective study. Int J Clin Oncol 25, 561–569 (2020). https://doi.org/10.1007/s10147-019-01606-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01606-8