Abstract
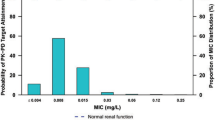
We evaluated the clinical and bacteriological efficacy of oral sitafloxacin (STFX) in clinically diagnosed community-acquired pneumonia (CAP) caused by Streptococcus pneumoniae. Additionally, we cultured these patient samples to test the minimal inhibitory concentrations (MICs) of levofloxacin (LVFX), moxifloxacin (MFLX), STFX, and penicillin G (PCG), as well as identified mutations in the quinolone resistance determinant regions (QRDRs) in LVFX-resistant strains. This study is a nested cohort from a prospective, multicenter clinical trial consisting of 139 patients with community-acquired pneumonia (CAP), from which 72 were included in this study. After diagnosis of CAP caused by S. pneumoniae, STFX (50 mg twice daily, or 100 mg once daily) was orally administered for 7 days. Sixty-five patient sputum samples were then cultured for MIC analysis. In a LVFX-resistant strain that was identified, mutations in the QRDRs of the gyrA, gyrB, parC, and parE genes were examined. Of 72 patients eligible for this study, S. pneumoniae was successfully cultured from the sputum of 65 patients, and only 7 patients were diagnosed by urinary antigen only. Clinical improvement of CAP was obtained in 65 of the 69 clinically evaluable patients (65/69, 94.2 %). Eradication of S. pneumoniae was observed in 62 patients of the 65 bacteriologically evaluable patients (62/65, 95.4 %). Additionally, STFX showed the lowest MIC distribution compared with LVFX, MFLX, and PCG, and no major adverse reactions were observed. STFX treatment in patients with CAP caused by S. pneumoniae was found to be highly effective both clinically (94.2 %) and bacteriologically (95.4 %).


Similar content being viewed by others
References
Appelbaum PC. Resistance among Streptococcus pneumoniae: implications for drug selection. Clin Infect Dis. 2002;34:1613–20.
File TM Jr. Clinical implications and treatment of multiresistant Streptococcus pneumoniae pneumonia. Clin Microbiol Infect. 2006;12(suppl 3):31–41.
Keating GM. Sitafloxacin: in bacterial infections. Drugs. 2011;71:731–44.
Saito A, Miki F, Oizumi K, Rikitomi N, Watanabe A, Koga H, et al. Clinical evaluation methods for new antimicrobial agents to treat respiratory infections: report of the Committee for the Respiratory System, Japan Society of Chemotherapy. J Infect Chemother. 1999;5:110–23.
National Committee for Clinical Laboratory Standards (2003) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. Sixth edition. NCCLS document M7-A6. NCCLS, Wayne, PA
Jorgensen JH, Weigel LM, Ferraro MJ, Swenson JM, Tenover FC. Activities of newer fluoroquinolones against Streptococcus pneumoniae clinical isolates including those with mutations in the gyrA, parC, and parE loci. Antimicrob Agents Chemother. 1999;43:329–34.
Saito A, Watanabe A, Aoki N, Niki Y, Kohno S, Kaku M, et al. Phase III double-blind comparative study of sitafloxacin versus tosufoxacin in patients with community-acquired pneumonia (in Japanese). Jpn J Chemother. 2008;56(suppl 1):49–62.
Kobayashi H, Watanabe A, Nakata K, Wada K, Niki Y, Kohono S. Double-blind comparative study of sitafloxacin versus levofloxacin in patients with respiratory tract infection (in Japanese). Jpn J Chemother. 2008;56(suppl 1):36–48.
Hawkey PM, Jones AM. The changing epidemiology of resistance. J Antimicrob Chemother. 2009;64(suppl 1):i3–10.
Ubukata K. Problems associated with high prevalence of multidrug-resistant bacteria in patients with community-acquired infections. J Infect Chemother. 2003;9:285–91.
Felmingham D, Reinert RR, Hirakata Y, Rodloff A. Increasing prevalence of antimicrobial resistance among isolates of Streptococcus pneumoniae from the PROTEKT surveillance study, and comparative in vitro activity of the ketolide, telithromycin. J Antimicrob Chemother. 2002;50(suppl 1):25–37.
Canton R, Morosini M, Enright MC, Morrissey I. Worldwide incidence, molecular epidemiology and mutations implicated in fluoroquinolone-resistant Streptococcus pneumoniae: data from the global PROTEKT surveillance programme. J Antimicrob Chemother. 2003;52:944–52.
Yokota S, Sato K, Kuwahara O, Habadera S, Tsukamoto N, Ohuchi H, et al. Fluoroquinolone-resistant Streptococcus pneumoniae strains occur frequently in elderly patients in Japan. Antimicrob Agents Chemother. 2002;46:3311–5.
Fuller JD, Low DE. A review of Streptococcus pneumoniae infection treatment failures associated with fluoroquinolone resistance. Clin Infect Dis. 2005;41:118–21.
Broskey J, Coleman K, Gwynn MN, McCloskey L, Traini C, Voelker L, et al. Efflux and target mutations as quinolones resistance mechanisms in clinical isolates of Streptococcus pneumoniae. J Antimicrob Chemother. 2000;45(suppl 1):95–9.
Richter SS, Heilmann KP, Beekmann SE, Miller NJ, Rice CL, Doern GV. The molecular epidemiology of Streptococcus pneumoniae with quinolone resistance mutations. Clin Infect Dis. 2005;40:225–35.
Jolley A, Andrews JM, Brenwald N, Wise R. The in vitro activity of a new highly active quinolone, DU-6859a. J Antimicrob Chemother. 1993;32:757–63.
Jones ME, Sahm DF, Martin N, Scheuring S, Heisig P, Thornsberry C, et al. Prevalence of gyrA, gyrB, parC, and parE mutations in clinical isolates of Streptococcus pneumoniae with decreased susceptibilities to different fluoroquinolones and originating from worldwide surveillance studies during 1997–1998 respiratory season. Antimicrob Agents Chemother. 2000;44:462–6.
Browne FA, Bozdogan B, Clark C, Kelly LM, Ednie L, Kosowska K, et al. Antipneumococcal activity of DK-507k, a new quinolone, compared with the activities of 10 other agents. Antimicrob Agents Chemother. 2003;47:3815–24.
Okumura R, Hirata T, Onodera Y, Hoshino K, Otani T, Yamamoto T. Dual-targeting properties of the 3-aminopyrrolidyl quinolones, DC-159a and sitafloxacin, against DNA gyrase and topoisomerase IV: contribution to reducing in vitro emergence of quinolone-resistant Streptococcus pneumoniae. J Antimicrob Chemother. 2008;62:98–104.
Yokota S, Ohkoshi Y, Fujii N. Susceptibility and bactericidal activity of 8 oral quinolones against conventional-fluoroquinolone-resistant Streptococcus pneumoniae clinical isolates. Diagn Microbiol Infect Dis. 2009;65:76–80.
Touyama M, Higa F, Nakasone C, Shinzato T, Akamine M, Haranaga S, et al. In vitro activity of sitafloxacin against clinical strains of Streptococcus pneumoniae with defined amino acid substitutions in QRDRs of gyrase A and topoisomerase IV. J Antimicrob Chemother. 2006;58:1279–82.
Hooper DC. Mechanisms of action of antimicrobials: focus on fluoroquinolones. Clin Infect Dis. 2001;32(suppl 1):S9–15.
Acknowledgments
We thank Daiichi Sankyo Co., Ltd., Tokyo, Japan, for providing editorial assistance. This work received financial support from Daiichi Sankyo Co., Ltd., Tokyo, Japan.
Conflict of interest
J.F. has served on speakers’ bureaus for GlaxoSmithKline, Abbott Japan, Boehringer Ingelheim, Pfizer, Astellas, Daiichi Sankyo, and Taisho Toyama. Y.N. has received a speaker’s honorarium and grant support from Daiichi-Sankyo. Y.T. has received a consultation fee from Daiichi Sankyo. A.W. is a consultant to Daiichi-Sankyo, Mitsubishi Tanabe Pharma Corporation, Toyama Chemical, and Otsuka Pharmaceutical. A.W. has received a speaker’s honorarium from MSD Japan, Glaxo SmithKline K.K., Shionogi & Co. Ltd., Daiichi-Sankyo, Taisho Toyama Pharmaceutical, Dainippon Sumitomo Pharma, and Pfizer Japan Inc. and grant support from Astellas Pharmaceutical, Kyorin Pharmaceutical, Shionogi & Co. Ltd., Taisho Pharmaceutical, Toyama Chemical, Daiichi Sankyo, Dainippon Sumitomo Pharmaceutical, Taiho Pharma, and Meiji Seika Pharma. S.H. has received a consultation fee from Daiichi Sankyo and has received a speaker’s honorarium from Daiichi-Sankyo.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Fujita, J., Niki, Y., Kadota, Ji. et al. Clinical and bacteriological efficacies of sitafloxacin against community-acquired pneumonia caused by Streptococcus pneumoniae: nested cohort within a multicenter clinical trial. J Infect Chemother 19, 472–479 (2013). https://doi.org/10.1007/s10156-012-0514-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10156-012-0514-4