Abstract
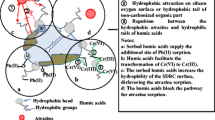
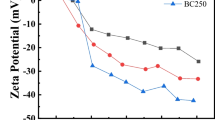
This study used excess sludge from a sewage treatment plant as raw material to extract humic acid (HA) and explore the ability of HA to adsorb Pb2+ from a solution. The effects of the adsorbent amount, solution pH, and co-existing cations on the adsorption process were investigated. The study showed that the humic acid derived from sludge (S-HA) surface had a loose, clustered texture. The S-HA surface contained many oxygen-containing functional groups, such as carboxyl groups, alcohol hydroxyl groups, and phenolic hydroxyl groups. The presence of co-existing cations, such as Na+, NH4+ and Ca2+, in the solution was not conducive to the adsorption of Pb2+ by S-HA. As the solution pH increased, the adsorption of Pb2+ by S-HA gradually increased. The process by which S-HA adsorbed Pb2+ conformed to a pseudo-second order kinetic model. Additionally, the overall adsorption rate was controlled by liquid membrane diffusion and intra-particle diffusion. The adsorption isotherm followed the Langmuir model, and the S-HA had a maximum adsorption capacity of 27.59 mg/g at 25 °C. The adsorption dynamics and thermodynamics results showed that the adsorption of Pb2+ by S-HA occurred via both physical adsorption and chemical adsorption processes.









Similar content being viewed by others
References
Andrew T (2019) Lead pollution of coastal sediments by ceramic waste. Mar Pollut Bull 138:171–176
Chinaro K, Robert L, Marissa SS, Rona B, Mary JB (2016) Evaluating the effectiveness of state specific lead-based paint hazard risk reduction laws in preventing recurring incidences of lead poisoning in children. Int J Hyg Envir Heal 219(1):110–117
Marie-Claude S, Pablo C, Arko S, Adele K, Asra S, Helmut H, Steven TS, Douglas R (2012) Epigenetics of early-life lead exposure and effects on brain development. Epigenomics 4(6):665–674
Kobyaa M, Demirbasb E, Senturka E, Ince M (2005) Adsorption of Heavy Metal Ions from Aqueous Solution by Activated Carbon Prepared from Apricot Stone. Bioresource Technol 96(13):1518–1521
Chen T, Zhou ZY, Han R, Meng R, Wang H, Lu W (2015) Adsorption of cadmium by biochar derived from municipal sewage sludge: impact factors and adsorption mechanism. Chemosphere 134:286–293
Gusiatin ZM, Kulikowska D, Klik B (2017) Suitability of humic substances recovered from sewage sludge to remedy soils from a former as mining area—a novel approach. J Hazard Mater 338:160–166
Dong B, Liu X, Dai L, Dai X (2013) Changes of heavy metal speciation during high-solid anaerobic digestion of sewage sludge. Bioresource Technol 131:152–158
Wei L, Qin K, Zhao Q et al (2017) Utilization of artificial recharged effluent for irrigation: pollutants’ removal and risk assessment. J Water Reuse Desal 7(1):77
Xue D, Huang X (2013) The impact of sewage sludge compost on tree peony growth and soil microbiological, and biochemical properties. Chemosphere 93(4):583–589
Manara P, Zabaniotou A (2012) Towards sewage sludge based biofuels via thermochemical conversion—a review. Renew Sust Energ Rev 16(5):2566–2582
Fukushima M, Chavoshi S, Bagheri M, Yetilmezsoy K, Samadi MT (2018) Analysis of branched-chain fatty acids in humic substances as indices for compost maturity by pyrolysis-gas chromatography/mass spectrometry with tetramethylammonium hydroxide (tmah-py-gc/ms). J Mater Cycles Waste 20(4):1999–2017
Melo BAGD, Motta FL, Santana MHA (2016) Humic acids: structural properties and multiple functionalities for novel technological developments. Mat Sci Eng C 62:967–974
Li SY, Li DY, Li JGX, Zhang BX, Li JJ (2017) Evaluation of humic substances during co-composting of sewage sludge and corn stalk under different aeration rates. Bioresource Technol 245:1299–1302
Zhen Y, Du MC, Jiang J (2016) Reducing capacities and redox potentials of humic substances extracted from sewage sludge. Chemosphere 144:902–908
Zhang C, Xu Y, Zhao M, Rong H, Zhang K (2018) Influence of inoculating white-rot fungi on organic matter transformations and mobility of heavy metals in sewage sludge based composting. J Hazard Mater 344:163–168
Lu M, Zhang Y, Zhou Y, Su Z, Liu B, Li G, Jiang T (2018) Adsorption-desorption characteristics and mechanisms of Pb(II) on natural vanadium, titanium-bearing magnetite-humic acid magnetic adsorbent. Powder Technol 344:947–958
Wang Q, Wang B, Lee XQ, Lehmann J, Bin G (2018) Sorption and desorption of Pb(II) to biochar as affected by oxidation and pH. Sci Total Environ 634:188–194
Gao YM, Borisover M, Cohen E, Rijn JV (2017) Accumulation of humic-like and proteinaceous dissolved organic matter in zero-discharge aquaculture systems as revealed by fluorescence EEM spectroscopy. Water Res 108:412–421
Zhang L, Sun X (2017) Addition of seaweed and bentonite accelerates the two-stage composting of green waste. Bioresource Technol 243:154–162
César P, Senesi N, Brunetti G, Mondelli D (2007) Evolution of the fulvic acid fractions during co-composting of olive oil mill wastewater sludge and tree cuttings. Bioresource Technol 98(10):1964–1971
Barje F, El Fels L, El Hajjouji H, Amir S, Winterton P, Hafidi M (2012) Molecular behaviour of humic acid-like substances during co-composting of olive mill waste and the organic part of municipal solid waste. Int Biodeter Biodegr 74:17–23
Wang X, Cui H, Shi J, Zhao X, Zhao Y, Wei Z (2015) Relationship between bacterial diversity and environmental parameters during composting of different raw materials. Bioresource Technol 198:395–402
Guo XX, Liu HT, Chang ZZ, Tao XP, Jin HM, Dong HM, Zhu ZP (2018) Review of humic substances developed in organic waste aerobic composting and its agronomic effect. J Ecol Rural Environ 34(6):489–498 (in Chinese)
Smidt E, Meissl K (2007) The applicability of Fourier transform infrared (FT-IR) spectroscopy in waste management. Waste Manage 27(2):268–276
Wu Q, Chen J, Clark M, Yu Y (2014) Adsorption of copper to different biogenic oyster shell structures. Appl Surf Sci 311:264–272
Garcia RG, Olguin MT, Colín-Cruz A, Romero-Guzmán ET (2012) Effect of the pH and temperature on the biosorption of lead (II) and cadmium (II) by sodium-modified stalk sponge of Zea mays. Environ Sci Pollut R 19(1):177–185
Unuabonah EI, Olu-Owolabi BI, Adebowale KO (2016) Competitive adsorption of metal ions onto goethite–humic acid-modified kaolinite clay. Int J Environ Sci Technol 13(4):1043–1054
Zhou Y, Zhang F, Tang L, Zhang J, Zeng G, Luo L, Liu Y, Wang P, Peng B, Liu X (2017) Simultaneous removal of atrazine and copper using polyacrylic acid-functionalized magnetic ordered mesoporous carbon from water: adsorption mechanism. Sci Rep UK 7:43831
Zeng G, Liu Y, Tang L, Yang G, Pang Y, Zhang Y, Zhou Y, Li Z, Li M, Lai M (2015) Enhancement of Cd (II) adsorption by polyacrylic acid modified magnetic mesoporous carbon. Chem Eng J 259:153–160
Sharma A, Lee BK (2014) Cd(II) removal and recovery enhancement by using acrylamide–titanium nanocomposite as an adsorbent. Appl Surf Sci 313:624–632
Hu XJ, Wang JS, Liu YG, Li X, Zeng GM, Bao ZL, Zeng XX, Chen AW, Long F (2011) Adsorption of chromium(VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: isotherms, kinetics and thermodynamics. J Hazard Mater 185:306–314
Wang H, Wang X, Ma J, Xia P, Zhao J (2017) Removal of cadmium (II) from aqueous solution: a comparative study of raw attapulgite clay and a reusable waste–struvite/attapulgite obtained from nutrient-rich wastewater. J Hazard Mater 329:66–76
Choudhary B, Paul D, Singh A, Gupta T (2017) Removal of hexavalent chromium upon interaction with biochar under acidic conditions: mechanistic insights and application. Environ Sci Pollut R 24(20):16786–16797
Choudhary B, Paul D (2018) Isotherms, kinetics and thermodynamics of hexavalent chromium removal using biochar. J Environ Chem Eng 6(2):2335–2343
Fan CH, Du B, Zhang YC, Ding S, Gao Y, Chang M (2017) Adsorption of lead on organo-mineral complexes isolated from loess in Northwestern China. J Geochem Explo 176:50–56
Zhang WH, Huang XC, Jia YM, Rees F, Tsang D, Qiu RL, Wang H (2017) Metal immobilization by sludge-derived biochar: roles of mineral oxides and carbonized organic compartment. Environ Geochem Health 39(2):379–389
An Y, Sun LP (2008) Study on optimum conditions of peat adsorbing Cu2+ and Pb2+ in heavy metal water. Humic Acid 5:29–33
Logan EM, Pulford ID, Cook GT, Mackenzie AB (1997) Complexation of Cu2+ and Pb2+ by peat and humic acid. Eur J Soil Sci 48(4):685–696
Feng YJ, Zhang ZH, Gao P, Su H, Yu YL, Ren NQ (2010) Adsorption behavior of EE2 (17α-ethinylestradiol) onto the inactivated sewage sluge: kinetics, thermodynamics and influence factors. J Hazard Mater 175:970–976
Acknowledgements
The authors acknowledge the financial support of the Natural Science Foundation of Gansu province (No. 20JR10RA236) and the University's Innovation Ability Improvement Project of Gansu Provincial office of Education (2019B-051).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dai, L., Zhao, W., Wei, B. et al. Adsorption of Pb2+ by insolubilized humic acid extracted from sewage sludge. J Mater Cycles Waste Manag 23, 1037–1047 (2021). https://doi.org/10.1007/s10163-021-01193-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-021-01193-9