Abstract
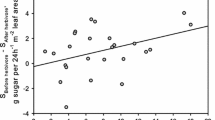
The disadvantage of induced defenses compared with constitutive defenses is the time during which a plant is vulnerable to herbivory before activation. There is obvious importance in determining the costs and benefits of induced defenses. Some plants produce extrafloral nectaries (EFNs), which attract ants that protect against herbivores, and induce EFNs and extrafloral nectar in response to leaf damage. To understand induction of indirect defense by ants, we investigated the induction and relaxation of extrafloral nectar secretion and EFN formation after artificial leaf damage in young Mallotus japonicus. Plants were grown under control or leaf damage conditions a greenhouse or in the field. Following artificial leaf damage, we assessed secretion of extrafloral nectar and the number of ant workers on plants. We measured the number of EFNs on each of seven leaves produced after leaf damage. Extrafloral nectar secretion was induced within 1 day following leaf damage, resulting in the attraction of numerous ant workers, and the extrafloral nectar secretion decreased to initial levels after 7 days. The number of EFNs was largest on the first leaf and smallest on the sixth leaf produced after leaf damage, but the total number of EFNs did not differ between treatments. Thus, M. japonicus rapidly induces extrafloral nectar secretion after leaf damage, followed by relaxation. Furthermore, following induction of EFNs on newly produced leaves, it may decrease the cost of induction by reducing the number of EFNs on leaves produced later.


Similar content being viewed by others
References
Agrawal AA (2000) Specificity of induced resistance in wild radish: causes and consequences for two specialist and two generalist caterpillars. Oikos 89:493–500
Agrawal AA, Strauss SS, Stout MJ (1999) Costs of induced response and tolerance to herbivory in male and female components of wild radish. Evol Int J org Evol 53:1093–1104
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc B (Methodological) 57:289–300
Bentley BL (1976) Plants bearing extrafloral nectaries and the associated ant community: interhabitat differences in the reduction of herbivore damage. Ecology 57:815–820
Bentley BL (1977) Extrafloral nectaries and protection by pugnacious bodyguards. Annu Rev Ecol Evol Syst 8:407–427
Brouat C, Mckey D, Bessière JM, Pascal L, Hossaert-Mckey M (2000) Leaf volatile compounds and the distribution of ant patrolling in an ant-plant protection mutualism: Preliminary results on Leonardoxa (Fabaceae: Caesalpinioideae) and Petalomyrmex (Formicidae: Formicinae). Acta Oecol 6:349–357
Conrath U, Beckers GJM, Flors V, Garcia-Agustin P, Jakab G, Mauch F, Newman M, Pieterse C, Poinssot B, Pozo M, Pugin A, Schaffrath U, Ton J, Wendehenne D, Zimmerli L, Mauch-Mani B (2006) Priming: getting ready for battle. Mol Plant Microbe In 19:1062–1071
De Witt TJ, Sih A, Wilson DS (1998) Costs and limits of phenotypic plasticity. Trends Ecol Evol 13:77–81
Frost CJ, Mescher MC, Carlson JE, De Moraes CM (2008) Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol 146:818–824
Gershenzon J (1994) Metabolic costs of terpenoid accumulation in higher plants. J Chem Ecol 20:1281–1328
Gomez S, van Dijk W, Stuefer JF (2010) Timing of induced resistance in a clonal plant network. Plant Biol 12:512–517
Heil M, Baldwin IT (2002) Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends Plant Sci 7:61–67
Heil M, McKey D (2003) Protective ant-plant interactions as model systems in ecological and evolutionary research. Ann Rev Ecol Evol Syst 34:425–553
Heil M, Fiala B, Linsenmair KE, Zotz G, Menke P, Maschwitz U (1997) Food body production in Macaranga triloba (Euphorbiaceae): a plant investment in anti-herbivore defence via symbiotic ant partners. J Ecol 85:847–861
Heil M, Fiala B, Baumann B, Linsenmair KE (2000) Temporal, spatial and biotic variations in extrafloral nectar secretion by Macaranga tanarius. Funct Ecol 14:749–757
Heil M, Koch T, Hilpert A, Fiala B, Boland W, Linsenmair KE (2001) Extrafloral nectar production of the ant-associated plat, Macaranga tanarius, is an induced, indirect, defensive response elicited by jasmonic acid. Proc Natl Acad Sci USA 98:1083–1088
Heil M, Greiner S, Meimberg H, Krüger R, Noyer JL, Heubl G, Linsenmair KE, Boland W (2004) Evolutionary change from induced to constitutive expression of an indirect plant resistance. Nature 430:205–208
Herms DA, Mattson WJ (1992) The dilemma of plants to grow or defend. Quarterly Rev Biol 67:283–335
Holland JN, Scott A, Horn CK (2009) Optimal defence theory predicts investment in resource in an ant-plant mutualism. J Ecol 97:89–96
Jones IM, Koptur S (2015) Dynamic extrafloral nectar production: the timing of leaf damage affects the defensive response in Senna mexicana var. chapmanii (Fabaceae). Ame J Bot 102:58–66
Karban R (2010) The ecology and evolution of induced resistance against herbivores. Funct Ecol 25:339–347
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
Koptur S (1989) Is extrafloral nectar production an inducible defense? In: Bock JH, Linhart YB (eds) Evolutionary Ecology of Plants. Westview, Boulder, pp 323–339
Lach L, Hobbs JR, Majer JD (2009) Herbivory-induced extrafloral nectar increases native and invasive ant worker survival. Popul Ecol 51:237–243
Mattiacci L, Rudelli S, Rocca BA, Genini S, Dorn S (2001) Systemically induced response of cabbage plants against a specialist herbivore, Pieris brassicae. Chemoecology 11:167–173
Mayer V, Schaber D, Hadacek F (2008) Volatiles of myrmecophytic Piper plants signal stem tissue damage to inhabiting Pheidole ant-partners. J Ecol 96:962–970
Metlen KL, Aschehoug ET, Callaway RM (2009) Plant genotype and induced responses affect resistance to herbivores on evening primrose (Oenothera Biennis). Ecol Entomol 31:20–31
Mondor EB, Addicott JF (2003) Conspicuous extrafloral nectaries are inducible in Vicia faba. Ecol Lett 6:495–497
Ness JH (2003) Catalpa bignonioides alters extrafloral nectar production after herbivory and attracts ant bodyguards. Oecologia 134:201–218
O’Dowd DJ (1982) Pearl bodies as ant food: an ecological role for some leaf emergences of tropical plants. Biotropica 14:40–49
Olson DM, Cortesero AM, Rains GC, Potter T, Lewis WJ (2009) Nitrogen and water affect direct and indirect plant systemic induced defense in cotton. Biol Cont 49:239–244
Pulice CE, Packer AA (2008) Simulated herbivory induces extrafloral nectary production in Prunus avium. Funct Ecol 22:801–807
Radhika V, Kost C, Bartram S, Heil M, Boland W (2008) Testing the optimal defence hypothesis for two indirect defences: extrafloral nectar and volatile organic compounds. Planta 228:449–457
Radhika V, Kost C, Mithöfer A, Boland W (2010) Regulation of extrafloral nectar secretion by jasmonates in lima bean is light dependent. Proc Natl Acad Sci USA 5:17228–17233
Schaller A (2008) Induced plant resistance to herbivory. Springer
Strauss SY, Rudgers JA, Lau JA, Irwin RE (2002) Direct and ecological costs of resistance to herbivory. Trends Ecol Evol 17:278–285
Wäckers FL, Zuber D, Wunderlin R, Keller F (2001) The effect of herbivory on temporal and spatial dynamics of foliar nectar production in cotton and castor. Ann Bot 87:365–370
Walters DR (2011) Plant defense. Warding off attack by pathogens, herbivores, and parasitic plants. Blackwell, Ames
Washitani I, Takenaka A (1987) Gap-detecting Mechanism in the seed germination of Mallotus japonicus (Thunb) Müell. Arg., a common pioneer tree of secondary succession in temperate Japan. Ecol Res 2:191–201
Wooley SC, Donaldson JR, Stevens MT, Gusse AC, Lindroth RL (2007) Extrafloral nectaries in aspen (Populus tremuloides): heritable genetic variation and herbivore-induced expression. Ann Bot 100:1337–1346
Yamawo A (2009) The defense strategies of Mallotus japonicus. Disertation, Okayama University of Science (in Japanese)
Yamawo A, Hada Y (2010) Effects of light on direct and indirect defences against herbivores of young plants of Mallotus japonicus demonstrate a trade-off between two indirect defence traits. Ann Bot 106:143–148
Yamawo A, Katayama N, Suzuki N, Hada Y (2012a) Plasticity in the expression of direct and indirect defence traits of young plants of Mallotus japonicus in relation to soil nutritional conditions. Plant Ecol 213:127–132
Yamawo A, Hada Y, Suzuki N (2012b) Variations in direct and indirect defenses against herbivores on young plants of Mallotus japonicus in relation to soil moisture conditions. J Plant Res 125:71–76
Yamawo A, Suzuki N, Tagawa J, Hada Y (2012c) Leaf ageing promotes the shift in defence tactics in Mallotus japonicus from direct to indirect defence. J Ecol 100:802–809
Yamawo A, Tagawa J, Hada Y, Suzuki N (2014) Different combinations of multiple defence traits in an extrafloral nectary-bearing plant growing under various habitat conditions. J Ecol 102:238–247
Yamawo A, Tokuda M, Katayama N, Yahara T, Tagawa J (2015) Ant-attendance in extrafloral nectar-bearing plants promotes growth and decreases the expression of traits related to direct defenses. Evol Biol 42:191–198
Yamawo A, Hada Y, Tagawa J (2017) Aggressiveness of ants attracted to the extrafloral nectary-bearing plant, Mallotus japonicus, and temporal fluctuations in their abundance. Entomol Sci 20:150–155
Young TP, Okello BD (1998) Relaxation of an induced defense after exclusion of herbivores: spines on Acacia drepanolobium. Oecologia 115:508–513
Young TP, Stanton ML, Christian CE (2003) Effects of natural and simulated herbivory on spine lengths of Acacia drepanolobium in Kenya. Oikos 101:171–179
Zong N, Wang CZ (2007) Larval feeding induced defensive response in tobacco: comparison of two sibling species of Helicoverpa with different diet breadths. Planta 226:215–224
Acknowledgements
We thank T. Yokota and S. Suematu for their assistance in the experiments. Thanks are also extended to R. Kishida for his advice on statistics. We express our gratitude to M. Kinoshita, N. Katayama, and K. Tanaka for their encouragement and advice provided throughout the study. This work was supported in part by a Research Fellowship from the Japan Society for the Promotion of Science (JSPS) for Young Scientists (234305) and a JSPS Grant-in-Aid for Young Scientists (B) (No. 15K18611).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamawo, A., Suzuki, N. Induction and relaxation of extrafloral nectaries in response to simulated herbivory in young Mallotus japonicus plants. J Plant Res 131, 255–260 (2018). https://doi.org/10.1007/s10265-017-0988-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-017-0988-3