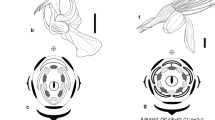
Abstract
Camoensia scandens is a papilionoid legume inserted in the core genistoid clade. It has large, crepuscular, scented flowers but the corolla is non-papilionaceous, which deviates from the pattern found in the subfamily. The vexillum has a folded claw, forming a tube, which is opposed to the androecium opening; all petals have yellow-gold crinkled margins. In addition, there is a long hypanthium, which stores a translucid liquid. The goal of this study is to elucidate the ontogenetic pathways that result in such a peculiar flower and the glands responsible for the sweet fragrance of the petals. Floral buds and flowers were processed for SEM, TEM and light microscopy analyses. Five sepals arise unidirectionally followed by five petals that initiate simultaneously. After the petals, 11 stamens emerge unidirectionally; a pair of adaxial stamens is opposite to the vexillum. In the intermediate developmental stages the sepals unite basally; the two adaxial sepals unite with each other to a greater extent than with the other sepals. The filaments are basally connate, forming a tube with an adaxial opening at the base. The carpel emerges concomitantly with the two abaxial antepetalous stamens. The long hypanthium forms from the outer floral organs (base of the sepals, petals, filaments) and is attached to the base of the stipe. The corolla is noticeable in the intermediate stages of development. The crinkled golden margins house scent glands formed of a secretory epidermis with secretory trichomes and secretory subepidermal cells. The odor is composed of neutral polysaccharides, nitrogenous substances and essential oils. An extensive nectariferous region is found on the inner surface of the hypanthial tube. The nectar is translucent, viscous and released through large pores. The comparison of our data with that of other genistoid flowers enabled discussions about the pollination and systematics of the group.








Similar content being viewed by others
References
Bisby FA (1981) Tribe 32. Genisteae (Adans.) Benth. (1865). In: Polhill RM, Raven PH (eds) Advances in Legume Systematics, vol 1. Kew Royal Botanic Gardens, London, pp 409–425
Boatwright JS, Savolainen V, van W Ben-Erik, Schutte-Vlok AL, Forest F, van der Bank M (2008) Systematic position of the anomalous genus Cadia and the phylogeny of the tribe Podalyrieae (Fabaceae). Syst Bot 33:133–147
Caissard J, Meekijjironenroj A, Baudino S, Anstett M (2004) Localization of production and emission of pollinator attractant on whole leaves of Chamaerops humilis (Arecaceae). Am J Bot 91:1190–1199
Cardoso D, Queiroz LP, Pennington RT, Lima HC, Fonty E, Wojciechowski MF, Lavin M (2012) Revisiting the phylogeny of papilionoid legumes: new insights from comprehensively sampled early-branching lineages. Am J Bot 99:1991–2013
Cardoso D, Pennington RT, Queiroz LP, Boatwright JS, Van Wyk B-E, Wojciechowski MF, Lavin M (2013) Reconstructing the deep branching relationships of the papilionoid legumes. South Afr J Bot 89:58–75
Castellanos C, Lewis GP, Banks H, Steeves R, Bruneau A (2015) Orphanodendron grandiflorum, uma nueva especie de leguminosa (Leguminosae: Papilionoideae) de los andes de Colombia. Brittonia 67:37–42
Castellanos C, Steeves R, Lewis GP, Bruneau A (2017) A settled sub-family for the orphan tree: The phylogenetic position of the endemic Colombian genus Orphanodendron in the Leguminosae. Brittonia 69:62–70
Citerne HL, Pennington RT, Cronk QCB (2006) An apparent reversal in floral symmetry in the legume Cadia is a homeotic transformation. PNAS 103:12017–12020
David R, Carde JP (1964) Coloration differentielle dês inclusions lipidique et terpeniques des pseudophylles du pine maritime au moyen du reactif Nadi. C R Biol 257:1338–1340
De Craene LR (2016) Meristic changes in flowering plants: how flowers play with numbers. Flora 221:22–37
Dudareva N, Pichersky E (2006) Biology of Floral Scent. Taylor & Francis Group, Boca Raton
Endress PK (1994) Diversity and evolutionary biology of tropical flowers. Cambridge University Press, New York
Fahn A (1979) Secretory tissues in plants. Academic Press, New York
Fahn A (1990) Plant anatomy, 4rd edn. Pergamon Press, Oxford
Feder N, O’Brien TP (1968) Plant microtechnique: some principles and methods. Am J Bot 55:123–142
Ferguson IK (1984) Pollen morphology and biosystematics of the subfamily Papilionoideae (Leguminosae). In: Grant WF (ed) Plant Biosyst, vol 2. Academic Press, Toronto, pp 377–394
Gerrits PO, Horobin RW (1991) The application of glycolmethacrylate in histotechnology; some fundamental principles. Department of Anatomy and Embryology, State University Groningen, Groningen
Gray A (1907) Structural botany or organography on the basis of morphology: to which is added the principles of taxonomy and phytography. American Book Company, New York
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Company Inc, New York
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137–138
Klitgaard BB (1999) Floral ontogeny in tribe Dalbergieae (Leguminosae: Papilionoideae): Dalbergia brasiliensis, Machaerium villosum sl., Platymiscium floribundum and Pterocarpus rotundifolius. Plant Syst Evol 219:1–25
Knuth P (1908) Handbook of flower pollination, vol II. Clarendon Press, Oxford
Kochanovski FJ, Paulino JV, Teixeira SP, Tozzi AMGA, Mansano VF (2018) Floral development of Hymenaea verrucosa: an ontogenetic approach to the unusual flower of Fabaceae subfamily Detarioideae. Bot J Linn Soc 1:46–58
Leite VG, Mansano VF, Teixeira SP (2014) Floral ontogeny in Dipterygeae (Fabaceae) reveals new insights into one of the earliest branching tribes in papilionoid legumes. Bot J Linn Soc 174:529–550
Leite VG, Teixeira SP, Mansano VF, Prenner G (2015) Floral development of the early branching papilionoid legume Amburana cearensis (Leguminosae) reveals rare and novel characters. Int J Plant Sci 176:94–106
Leite VG, Mansano VF, Pansarin E, Teixeira SP (2019) Presence of the anther gland is a key feature in the pollination of the early-branching papilionoids Dipteryx alata and Pterodon pubescens (Leguminosae). Plant Biol 21:1016–1023
Leppik EE (1966) Floral evolution and pollination in the Leguminosae. Ann Bot Fenn 3:299–308
Lewis G, Schrire B, Mackinder B, Lock M (2005) Legumes of the world. Royal Botanic Gardens, Kew
LPWG (2017) A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 66:44–77
Mansano VF, Teixeira SP (2008) Floral anatomy of the Lecointea clade (Leguminosae, Papilionoideae, Swartzieae sensu lato). Plant Syst Evol 273:201–209
MansanoVF, Tucker SC, Tozzi AMGA (2002) Floral ontogeny of Lecointea, Zollernia, Exostiles and Harleyodendron (Leguminosae: Papilionoideae: Swartzieae s.l.). Am J Bot 89:1553–1569
Marinho CR, Souza CD, Teixeira SP (2014) Scent glands in legume flowers. Plant Biol 16:215–226
McMahon MM, Hufford L (2005) Evolution and development in the amorphoid clade (Amorpheae: Papilionoideae: Leguminosae): petal loss and dedifferentiation. Int J Plant Sci 166:383–396
Moço MCC, Mariath JEA (2009) Comparative floral ontogeny in Adesmia (Leguminosae: Papilionoideae: Dalbergieae). Aust J Bot 57:65–75
Naghiloo S, Dadpour MR, Movafeghi A (2012) Floral ontogeny in Astragalus compactus (Leguminosae: Papilionoideae: Galegeae): variable occurrence of bracteoles and variable patterns of sepal initiation. Planta 235:793–805
Nilsson LA (1992) Orchid pollination biology. Trends Ecol Evol 7:255–259
O’Brien TP, Feder N, McCcully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373
Oliveira MIB, Sigrist MR (2008) Fenologia reprodutiva, polinização e reprodução de Dipteryx alata Vogel (Leguminosae-Papilionoideae) em Mato Grosso do Sul, Brasil. Rev Bras de Bot 31:195–207
Oliveira PE, Gibbs PE, Barbosa AA (2004) Moth pollination of woody species in the Cerrados of Central Brazil: a case of so much owed to so few? Plant Syst Evol 245:41–54
Paulino JV, Groppo M, Teixeira SP (2011) Floral developmental morphology of three Indigofera species (Leguminosae) and its systematic significance within Papilionoideae. Plant Syst Evol 292:165–176
Paulino JV, Mansano FV, Teixeira SP (2013) Elucidating the unusual floral features of Swartzia dipetala (Fabaceae). Bot J Linn Soc 173:303–320
Paulino JV, Mansano FV, Prenner G (2016) Evidence for division of labor and division of function related to the pollen release in Papilionoideae (Leguminosae) with a heteromorphic androecium. Int J Plant Sci 177:590–607
Pearse AGE (1985) Histochemistry: theoretical and applied. Churchill Livingston, Edinburgh, UK
Pedersoli GD, Paulino JV, Leite VG, Teixeira SP (2010) Elucidating enigmatic floral issues in Copaifera langsdorffii Desf. (Leguminosae, Caesalpinioideae). Int J Plant Sci 171:834–846
Pennington CH, Stirton CH, Schire BD (2005) Tribe Sophoreae. In: Lewis G, Schrire B, Mackinder B, Lock M (eds) Legumes of the world. Royal Botanic Gardens, Kew, pp 227–249
Polhill RM (1981a) Sophoreae. In: Polhill RM, Raven PH (eds) Advances in legume systematics, part 1. Royal Botanic Gardens, Kew, pp 213–230
Polhill RM (1981b) Papilionoideae. In: Polhill RM, Raven PH (eds) Advances in legume systematics, part 1, vol 1. Royal Botanic Gardens, Kew, pp 191–409
Prenner G (2004a) The asymmetric androecium in Papilionoideae (Leguminosae): definition, occurrence, and possible systematic value. Int J Plant Sci 165:499–510
Prenner G (2004b) New aspects in floral development of Papilionoideae: initiated but suppressed bracteoles and variable initiation of sepals. Ann Bot 93:537–545
Prenner G (2004c) Floral development in Daviesia cordata (Leguminosae: Papilionoideae: Mirbelieae) and its systematic implications. Aust J Bot 52:285–291
Prenner G (2013a) Papilionoid inflorescences revisited (Leguminosae: Papilionoideae). Ann Bot 112:1567–1576
Prenner G (2013b) Flower development in Abrus precatorius (Leguminosae: Papilionoideae: Abreae) and a review of androecial characters in Papilionoideae. South Afr J Bot 89:210–218
Rodríguez-Riaño T, Ortega-Olivencia A, Devesa JA (1999) Types of androecium in the Fabaceae of SW Europe. Ann Bot 83:109–116
Sattler R (1978) ‘Fusion’ and ‘Continuity’ in floral morphology. Notes Roy Bot Gard Edinburgh 36:397–405
Simpson MG (2006) Plant Systemat. Elsevier/Academic Press, Amsterdam
Teixeira SP, Ranga NT, Tucker SC (2009) Inflorescence and floral development of Dahlstedtia species (Leguminosae: Papilionoideae: Millettieae). Flora 204:769–781
Teixeira SP, Marinho CR, Paulino JV (2014) A flor: aspectos morfofuncionais. In: Rech AR, Agostini K, Oliveira PE, Machado IC (eds) Biologia da Polinização, vol 1. Projeto Cultural, Rio de Janeiro, pp 45–69
Tucker SC (1987) Floral initiation and development in legumes. In: Stirton CH (ed) Advances in legume systematics, part 3. Royal Botanic Gardens, Kew, pp 183–239
Tucker SC (1988) Dioecy in Bauhinia resulting from organ suppression. Am J Bot 75:205–224
Tucker SC (1992) The role of floral development in studies of legume evolution. Can J Bot 70:692–700
Tucker SC (1994) Floral ontogeny in Sophoreae (Leguminosae: Papilionoideae): II. sensu lato (Sophora group). Am J Bot 81:368–380
Tucker SC (1997) Floral evolution, development, and convergence: the hierarchical-significance hypothesis. Int J Plant Sci 156:143–161
Tucker SC (2000) Floral development in tribe Detarieae (Leguminosae: Caesalpinioideae): Amherstia, Brownea, and Tamarindus. Am J Bot 87:1385–1407
Tucker SC (2002) Floral ontogeny in Sophoreae (Leguminosae: Papilionoideae). III. Radial symmetry and random petal aestivation in Cadia purpurea. Am J Bot 89:748–757
Tucker SC (2003a) Floral development in legumes. Plant Physiol 131:911–926
Tucker SC (2003b) Floral ontogeny in Swartzia (Leguminosae: Papilionoideae: Swartzieae): distribution and role of the ring meristem. Am J Bot 90:1271–1292
Tucker SC (2003c) Comparative floral ontogeny in Detarieae (Leguminosae: Caesalpinioideae). III. Adaxially initiated whorls in Julbernardia and Sindora. Int J Plant Sci 164:275–286
Vidal BC (1970) Dichroism in collagen bundles stained with xylidine Ponceau 2R. Ann Histochim 15:289–296
Vogel S (1969) Chiropterophilie in der neotropischen Flora. Neue Mitteilungen II Flora 158:185–222
Vogel S (1983) Ecophysiology of zoophilic pollination. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology III. Encyclopedia of plant physiology, vol 12C. Springer-Verlag Berlin, Heidelberg, pp 559–624
Vogel S (1990) The role of scent glands in pollination: on the structure and function of osmophores. Smithsonian Institution Libraries, Washington, (Translated by S.S. Renner)
von Hegnauer R (1996) Chemotaxonomie der Pflanzen Band XIb-1 Leguminosae Teil 2: Caesalpinoideae und Mimosoideae. Birkhäuser Verlag, Basel
Westerkamp C, Weber A (1997) Keel flowers of the Polygalaceae and Fabaceae: a functional comparison. Bot J Linn Soc 129:207–221
Wojciechowski MF, Lavin M, Sanderson MJ (2004) A phylogeny of legumes (Leguminosae) based on analysis of the plastid matK gene resolves many well supported subclades within the family. Am J Bot 91:1845–1861
Yakovlev GP (1972) A contribution to the system of the order Fabales Nakai (Leguminales Jones). Bot Zhurn 57:585–595
Acknowledgements
Research supported by CNPq (process numbers 421121/2016-5 and 154529/2016-8 and 302806/2019-9), CAPES (finance code 001) and FAPERJ (process number E-26/010.100998/2018). We are grateful to Rodrigo Ferreira Silva (FFCLRPUSP), Maria Dolores Seabra Ferreira, José Augusto Maulin (FMRPUSP), Edimárcio da Silva Campos (FCFRP/USP), and João Paulo Basso-Alves (JBRJ) for technical assistance, Bruno Garcia Simões Favaretto for the drawings composing Fig. 1 and to Dewey Litwiller (University of Saskatchewan) and Elettra Greene for revision of the English text, Dr Louis Ronse De Craene and an anonymous reviewer for valuable comments on the manuscript.
Author information
Authors and Affiliations
Contributions
VGL, VFM, FG and JVP performed the experiments and analyzed the data. SPT and VFM conceived and designed the experiments. All authors wrote the manuscript.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Leite, V.G., Teixeira, S.P., Godoy, F. et al. Resolving the non‐papilionaceous flower of Camoensia scandens, a papilionoid legume of the core genistoid clade: development, glands and insights into the pollination and systematics of the group. J Plant Res 134, 823–839 (2021). https://doi.org/10.1007/s10265-021-01293-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-021-01293-5