Abstract
Ralstonia solanacearum is the causal organism of bacterial wilt of more than 200 species representing 50 families of plants in tropical, subtropical, and warm temperate regions in the world. Traditionally classified into five races based on differences in host range, R. solanacearum has also been grouped into six biovars on the basis of biochemical properties. With recent developments in molecular biology, various DNA-based analyses have been introduced and used to confirm that this binary system does not completely represent the diversity within R. solanacearum strains. Therefore, a new hierarchical classification scheme has been suggested, which defines R. solanacearum as a species complex and reorganized the concept of the species as a monophyletic cluster according to a phylogenetic analysis based on genomic sequence data. Here we discuss the current bacterial wilt situation and genetic relationships based on the recent classification system of Japanese R. solanacearum strains as well as worldwide strains. We also review the genetic, biochemical, and pathological characteristics of R. solanacearum strains, in particular, those affecting potato and Zingiberaceae plants as distinctly important pathogens in relation to continuously problematic and recent emergent diseases in Japan.

Similar content being viewed by others
References
Almeida NF, Yan S, Cai R, Clarke CR, Morris CE, Schaad NW, Schuenzel EL, Lacy GH, Sun X, Jones JB, Castillo JA, Bull CT, Leman S, Guttman DS, Setubal JC, Vinatzer BA (2010) PAMDB, a multilocus sequence typing and analysis database and website for plant-associated microbes. Phytopathology 100:208–215
Boshou L (2005) A broad review and perspective on breeding for resistance to bacterial wilt. In: Allen C, Prior P, Hayward AC (eds) Bacterial wilt disease and the Ralstonia solanacearum species complex. APS Press, St. Paul, pp 225–238
Buddenhagen I, Kelman A (1964) Biological and physiological aspects of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol 2:203–230
Castillo JA, Greenberg JT (2007) Evolutionary dynamics of Ralstonia solanacearum. Appl Environ Microbiol 73:1225–1238
Cook D, Sequeira L (1994) Strain differentiation of Pseudomonas solanacearum by molecular genetic methods. In: Hayward AC, Hartman GL (eds) Bacterial wilt: the disease and its causative agent, Pseudomonas solanacearum. CAB International, Wallingford, pp 77–93
Cook D, Barlow E, Sequeira L (1989) Genetic diversity of Pseudomonas solanacearum: detection of restriction fragment length polymorphisms with DNA probes that specify virulence and the hypersensitive response. Mol Plant Microbe Interact 2:113–121
Denny TP, Hayward AC (2001) Gram-negative bacteria: Ralstonia. In: Schaad NW, Jones JB, Chun W (eds) Laboratory guide for identification of plant pathogenic bacteria, 3rd edn. APS Press, St. Paul, pp 151–174
Eden-Green SJ, Sastraatmadja H (1990) Blood disease of banana present in Java. FAO Plant Protect Bull 38:49–50
Elphinstone JG (2005) The current bacterial wilt situation: a global overview. In: Allen C, Prior P, Hayward AC (eds) Bacterial wilt disease and the Ralstonia solanacearum species complex. APS Press, St. Paul, pp 9–28
Fegan M, Prior P (2005) How complex is the “Ralstonia solanacearum species complex”? In: Allen C, Prior P, Hayward AC (eds) Bacterial wilt disease and the Ralstonia solanacearum species complex. APS Press, St. Paul, pp 449–461
Fegan M, Taghavi M, Sly LI, Hayward AC (1998) Phylogeny, diversity and molecular diagnostics of Ralstonia solanacearum. In: Prior P, Allen C, Elphinstone J (eds) Bacterial wilt disease: molecular and ecological aspects. Springer, Berlin, pp 19–33
Gillings MR, Fahy P (1993) Genetic diversity of Pseudomonas solanacearum biovar 2 and N2 assessed using restriction endonuclease analysis of total genomic DNA. Plant Pathol 42:744–753
Gillings MR, Fahy P (1994) Genomic fingerprinting: towards a united view of the Pseudomonas solanacearum species complex. In: Hayward AC, Hartman GL (eds) Bacterial wilt: The disease and its causative agent, Pseudomonas solanacearum. CAB International, Wallingford, pp 95–112
Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM (2007) DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57:81–91
Hayward AC (1964) Characteristics of Pseudomonas solanacearum. J Appl Bacteriol 27:265–277
Hayward AC (1991) Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol 29:65–87
Hayward AC (1994a) The hosts of Pseudomonas solanacearum. In: Hayward AC, Hartman GL (eds) Bacterial wilt: the disease and its causative agent, Pseudomonas solanacearum. CAB International, Wallingford, pp 9–24
Hayward AC (1994b) Systematics and phylogeny of Pseudomonas solanacearum and related bacteria. In: Hayward AC, Hartman GL (eds) Bacterial wilt: the disease and its causative agent, Pseudomonas solanacearum. CAB International, Wallingford, pp 123–135
Hayward AC, Pegg KG (2013) Bacterial wilt of ginger in Queensland: reappraisal of a disease outbreak. Aust Plant Pathol 42:235–239
Hayward AC, Moffet ML, Pegg KG (1967) Bacterial wilt of ginger in Queensland. Qld J Agric Anim Sci 24:1–5
Hayward AC, Elphinstone JG, Caffier D, Janse J, Stefani E, French ER, Wright AJ (1998) Round table on bacterial wilt (brown rot) of potato. In: Prior P, Allen C, Elphinstone J (eds) Bacterial wilt disease: molecular and ecological aspects. Springer, Berlin, pp 420–430
He LY (1986) Bacterial wilt in the People’s Republic of China. In: Persley GJ (ed) Bacterial wilt disease in Asia and the South Pacific. Australian Centre for International Agricultural Research (ACIAR) Proc No. 13, Canberra, pp 40–48
He LY, Sequeira L, Kelman A (1983) Characteristics of strains of Pseudomonas solanacearum from China. Plant Dis 67:1357–1361
Hong JC, Norman DJ, Reed DL, Momol MT, Jones JB (2012) Diversity among Ralstonia solanacearum strains isolated from the southeastern US. Phytopathology 102:924–936
Horita M, Ooshiro A (2002) Genetic diversity of Ralstonia solanacearum strains isolated from potato and balsam pear in Okinawa Island. Bact Wilt Newsl 17:23
Horita M, Tsuchiya K (2000) Comparative analysis of Japanese and foreign strains of Ralstonia solanacearum based on 16S ribosomal RNA gene sequences. J Gen Plant Pathol 66:132–137
Horita M, Tsuchiya K (2001) Genetic diversity of Japanese strains of Ralstonia solanacearum. Phytopathology 91:399–407
Horita M, Tsuchiya K (2009) Current status and future prospects of the classification system for the bacterial wilt pathogen Ralstonia solanacearum species complex (in Japanese). Jpn J Phytopathol 75:297–306
Horita M, Tsuchiya K (2012) MAFF microorganism genetic resource manual no. 12 (ver. 2) Ralstonia solanacearum (in Japanese). National Institute of Agrobiological Sciences, Tsukuba, pp 1–32
Horita M, Yano K, Tsuchiya K (2004) PCR-based specific detection of Ralstonia solanacearum race 4 strains. J Gen Plant Pathol 70:278–283
Horita M, Tsuchiya K, Ooshiro A (2005) Characteristics of Ralstonia solanacearum biovar N2 strains in Asia. J Phytopathol 153:209–213
Horita M, Suga Y, Ooshiro A, Tsuchiya K (2010) Analysis of genetic and biological characters of Japanese potato strains of Ralstonia solanacearum. J Gen Plant Pathol 76:196–207
Jaunet TX, Wang JF (1999) Variation in genotype and aggressiveness of Ralstonia solanacearum race 1 isolated from tomato in Taiwan. Phytopathology 89:320–327
Jeong Y, Kim J, Kang Y, Lee S, Hwang I (2007) Genetic diversity and distribution of Korean isolates of Ralstonia solanacearum. Plant Dis 91:1277–1287
Katayama K, Kimura S (1986) Ecology and protection of bacterial wilt of potato: 1. Ecology and strains of Pseudomonas solanacearum (in Japanese with English summary). Bull Nagasaki Agric Forest Exp Stn 14:1–30
Kumar A, Anandaraj M, Sarma YR (2005) Rhizome solarization and microwave treatment: ecofriendly methods for disinfecting ginger seed rhizomes. In: Allen C, Prior P, Hayward AC (eds) Bacterial wilt disease and the Ralstonia solanacearum species complex. APS Press, St. Paul, pp 185–195
Lapage SP, Sneath PHA, Lessel EF, Skerman VBD, Seeliger HPR, Clark WA (eds) (1992) International code of nomenclature of bacteria. ASM Press, Washington (DC)
Lewis Ivey ML, McSpadden Gardener BB, Opina N, Miller SA (2007) Diversity of Ralstonia solanacearum infecting eggplant in the Philippines. Phytopathology 97:1467–1475
Liu Y, Kanda A, Yano K, Kiba A, Hikichi Y, Aino M, Kawaguchi A, Mizoguchi S, Nakaho K, Shiomi H, Takikawa Y, Ohnishi K (2009) Molecular typing of Japanese strains of Ralstonia solanacearum in relation to the ability to induce a hypersensitive reaction in tobacco. J Gen Plant Pathol 75:369–380
Lum KY (1973) Cross inoculation studies of Pseudomonas solanacearum from ginger. MARDI Res Bull 1:15–21
Mahbou Somo Toukam G, Cellier G, Wicker E, Guilhaud C, Kahane R, Allen C, Prior P (2009) Broad diversity of Ralstonia solanacearum strains in Cameroon. Plant Dis 93:1123–1130
Maiden MC (2006) Multilocus sequence typing of bacteria. Annu Rev Microbiol 60:561–588
Morita Y, Yano K, Tsuchiya K, Kawada Y (1996) Bacterial wilt of Curcuma alismatifolia caused by Pseudomonas solanacearum (in Japanese). Proc Assoc Pl Protec Shikoku 31:1–6
Narita T (1958) Studies on the bacterial diseases of potato in Hokkaido (in Japanese with English summary). Rep Hokkaido Pref Agric Exp Stn 8:1–80
Norman DJ, Zapata M, Gabriel DW, Duan YP, Yuen JMF, Mangravita-Novo A, Donahoo RS (2009) Genetic diversity and host range variation of Ralstonia solanacearum strains entering North America. Phytopathology 99:1070–1077
Nouri S, Bahar M, Fegan M (2009) Diversity of Ralstonia solanacearum causing potato bacterial wilt in Iran and the first record of phylotype II/biovar 2T strains outside South America. Plant Pathol 58:243–249
Okabe N (1965) Strains of Pseudomonas solanacearum E. F. Smith (in Japanese). Ann Phytopath Soc Japan 31:152–158
Ooshiro A (2008) Control of bacterial wilt by Geranium carolinianum L. (in Japanese). Plant Protect 62:90–95
Ozaki K, Kimura T (1992) Grouping of Pseudomonas solanacearum on the basis of pathogenicity to Solanum plants (in Japanese). Bull Chugoku Natl Agric Exp Stn 10:49–58
Palleroni NJ, Doudoroff M (1971) Phenotypic characterization and deoxyribonucleic acid homologies of Pseudomonas solanacearum. J Bacteriol 107:690–696
Pegg KG, Moffett ML (1971) Host range of the ginger strain of Pseudomonas solanacearum in Queensland. Austral J Exp Agric Anim Hus 11:696–698
Pérez-Losada M, Browne EB, Madsen A, Wirth T, Viscidi RP, Crandall KA (2006) Population genetics of microbial pathogens estimated from multilocus sequence typing (MLST) data. Infect Genet Evol 6:97–112
Poussier S, Vandewalle P, Luisetti J (1999) Genetic diversity of African and worldwide strains of Ralstonia solanacearum as determined by PCR-restriction fragment length polymorphism analysis of the hrp gene region. Appl Environ Microbiol 65:2184–2194
Poussier S, Prior P, Luisetti J, Hayward C, Fegan M (2000a) Partial sequencing of the hrpB and endoglucanase genes confirms and expands the known diversity within the Ralstonia solanacearum species complex. Syst Appl Microbiol 23:479–486
Poussier S, Trigalet-Demery D, Vandewalle P, Goffinet B, Luisetti J, Trigalet A (2000b) Genetic diversity of Ralstonia solanacearum as assessed by PCR-RFLP of the hrp gene region, AFLP and 16S rRNA sequence analysis, and identification of an African subdivision. Microbiology 146:1679–1692
Prior P, Fegan M (2005) Recent developments in the phylogeny and classification of Ralstonia solanacearum. Acta Hortic 695:127–136
Quinon VL, Aragaki M, Ishii M (1964) Pathogenicity and serological relationship of three strains of Pseudomonas solanacearum in Hawaii. Phytopathology 54:1096–1099
Remenant B, Coupat-Goutaland B, Guidot A, Cellier G, Wicker E, Allen C, Fegan M, Pruvost O, Elbaz M, Calteau A, Salvignol G, Mornico D, Mangenot S, Barbe V, Medigue C, Prior P (2010) Genomes of three tomato pathogens within the Ralstonia solanacearum species complex reveal significant evolutionary divergence. BMC Genom 11:379
Remenant B, de Cambiaire J-C, Cellier G, Jacobs JM, Mangenot S, Barbe V, Lajus A, Vallenet D, Medigue C, Fegan M, Allen C, Prior P (2011) Ralstonia syzygii, the blood disease bacterium and some Asian R. solanacearum strains form a single genomic species despite divergent lifestyles. PLoS One 6:e24356
Schaad NW, Frederick RD, Shaw J, Schneider WL, Hickson R, Petrillo MD, Luster DG (2003) Advances in molecular-based diagnostics in meeting crop biosecurity and phytosanitary issues. Annu Rev Phytopathol 41:305–324
Suga Y (2000) Ecology and control of bacterial wilt of potato (in Japanese). Phytopathol Soc Jpn Soilborne Dis Workshop Rep 20:174–179
Suga Y, Horita M, Umekita M, Furuya N, Tsuchiya K (2013) Pathogenic characters of Japanese potato strains of Ralstonia solanacearum. J Gen Plant Pathol 79:110–114
Swanson JK, Yao J, Tans-Kersten J, Allen C (2005) Behavior of Ralstonia solanacearum race 3 biovar 2 during latent and active infection of geranium. Phytopathology 95:136–143
Taghavi M, Hayward C, Sly LI, Fegan M (1996) Analysis of the phylogenetic relationships of strains of Burkholderia solanacearum, Pseudomonas syzygii, and the Blood disease bacterium of banana based on 16S rRNA sequences. Int J Syst Bacteriol 46:10–15
Thwaites R, Mansfield J, Eden-Green S, Seal S (1999) RAPD and rep PCR-based fingerprinting of vascular bacterial pathogens of Musa spp. Plant Pathol 48:121–128
Titatarn V (1986) Bacterial wilt in Thailand. In: Persley GJ (ed) Bacterial wilt disease in Asia and the South Pacific. Australian Centre for International Agricultural Research (ACIAR) Proc No. 13, Canberra, pp 65–67
Tsuchiya K (2008) Occurrence and spread of bacterial wilt diseases of Zingiberaceae plants caused by foreign strains (in Japanese). Plant Protect 62:72–75
Tsuchiya K, Yano K, Horita M, Morita Y, Kawada Y, d’Ursel CM (1999) Occurrence of bacterial wilt of ginger in Japan (abstract in Japanese). Ann Phytopathol Soc Jpn 65:363
Tsuchiya K, Yano K, Horita M, Morita Y, Kawada Y, d’Ursel CM (2005) Occurrence and epidemic adaptation of new strains of Ralstonia solanacearum associated with Zingiberaceae plants under agro-ecosystem in Japan. In: Allen C, Prior P, Hayward AC (eds) Bacterial wilt disease and the Ralstonia solanacearum species complex. APS Press, St. Paul, pp 463–469
Uematsu T, Chutenchitt S, Karnjanarat S, Vivithajinda S, Nabheerong N, Benjathikul S, Nilmanee S, Dhirabhava W, Buangsuwon D (1983) Bacterial diseases on economic crops in Thailand. Tropical Agricultural Research Center, Ministry of Agriculture, Forestry, and Fisheries. Japan and Department of Agriculture, Ministry of Agriculture and Cooperatives, Thailand, pp 1–266
Vaneechoutte M, Kämpfer P, De Baere T, Falsen E, Verschraegen G et al (2004) Wautersia gen. nov., a novel genus accommodating the phylogenetic lineage including Ralstonia eutropha and related species, and proposal of Ralstonia [Pseudomonas] syzygii (Roberts et al. 1990) comb. nov. Int J Syst Evol Microbiol 54:317–327
Villa JE, Tsuchiya K, Horita M, Natural M, Opina N, Hyakumachi M (2005) Phylogenetic relationships of Ralstonia solanacearum species complex strains from Asia and other continents based on 16S rDNA, endoglucanase, and hrpB sequences. J Gen Plant Pathol 71:39–46
Waki T, Horita M, Kurose D, Mulya K, Tsuchiya K (2013) Genetic diversity of Zingiberaceae plant isolates of Ralstonia solanacearum in the Asia-Pacific Region. JARQ 47:283–294
Wicker E, Lefeuvre P, de Cambiaire J-C, Lemaire C, Poussier S, Prior P (2012) Contrasting recombination patterns and demographic histories of the plant pathogen Ralstonia solanacearum inferred from MLSA. ISME J 6:961–974
Xu J, Pan ZC, Prior P, Xu JS, Zhang Z, Zhang H, Zhang LQ, He LY, Feng J (2009) Genetic diversity of Ralstonia solanacearum strains from China. Eur J Plant Pathol 125:641–653
Xue Q-Y, Yin Y-N, Yang W, Heuer H, Prior P, Guo J-H, Smalla K (2011) Genetic diversity of Ralstonia solanacearum strains from China assessed by PCR-based fingerprints to unravel host plant- and site-dependent distribution patterns. FEMS Microbiol Ecol 75:507–519
Yabuuchi E, Kosako Y, Yano I, Hotta H, Nishiuchi Y (1996) Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen. nov.: proposal of Ralstonia pickettii (Ralston, Palleroni and Doudoroff 1973) comb. nov., Ralstonia solanacearum (Smith 1896) comb. nov. and Ralstonia eutropha (Davis 1969) comb. nov. Microbiol Immunol 39:897–904
Yano K, Kawada Y, Tsuchiya K, Horita M (2005) First report of bacterial wilt of mioga (Zingiber mioga) caused by Ralstonia solanacearum in Japan (in Japanese with English summary). Jpn J Phytopathol 71:179–182
Yano K, Kawada Y, Horita M, Hikichi Y, Tsuchiya K (2011) Phylogenetic discrimination and host ranges of Ralstonia solanacearum isolates from Zingiberaceae plants (in Japanese with English summary). Jpn J Phytopathol 77:88–95
Yu Q, Alvarez AM, Moore PH, Zee F, Kim MS, de Silva A, Hepperly PR, Ming R (2003) Molecular diversity of Ralstonia solanacearum isolated from ginger in Hawaii. Phytopathology 93:1124–1130
Zehr EI (1969) Bacterial wilt of ginger in the Philippines. Philipp Agricst 53:224–227
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Horita and K. Tsuchiya have contributed equally to preparation of the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10327_2014_537_MOESM1_ESM.ppt
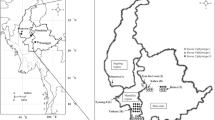
Distribution and presumable invasion routes of bacterial wilt pathogen of Zingiberaceae plants in Japan. Yellow and blue arrowheads indicate presumable invasion routes of the pathogen representing DNA type I (yellow circle) and type II (blue circle) fingerprints for strains suggested to originate from Thailand and China, respectively (Tsuchiya 2008; Tsuchiya et al. 2005). These strains were first disseminated to Kochi Prefecture through latently infected seed rhizomes, and the first outbreak of the disease occurred in a curcuma field in 1995 (type I) and subsequently in a ginger field in 1997 (type II). Thereafter, the disease has been found continuously in several prefectures since 2005. The yellow dotted arrowheads indicate suspected routes of spread. The number in parentheses near the prefecture name indicates the year when the disease was first found there. (PPT 176 kb)
Rights and permissions
About this article
Cite this article
Horita, M., Tsuchiya, K., Suga, Y. et al. Current classification of Ralstonia solanacearum and genetic diversity of the strains in Japan. J Gen Plant Pathol 80, 455–465 (2014). https://doi.org/10.1007/s10327-014-0537-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-014-0537-z