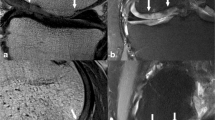
Abstract
Objectives
To test PEEK implant-associated MRI artifacts, a method for blinding MRI readers, the repeatability of cartilage thickness measures before and 6 weeks after high tibial osteotomy (HTO), and the sensitivity to change of cartilage thickness 12 months after HTO.
Materials and methods
Ten patients underwent HTO using a PEEK implant and 3 T-MRI before, 6 weeks and 12 months after surgery. Masks were applied to hide implant visibility on 48 MRI pairs, which were assessed by 7 readers (blinded to time). One blinded reader measured femorotibial cartilage thickness from masked MRIs.
Results
No artifacts were produced. Readers were unable to identify scans by time greater than by chance. Cartilage thickness before and 6 weeks after surgery was not significantly different and indicated excellent repeatability. Medial cartilage thickness increases 12 M postoperatively approached statistical significance (p = 0.06), with no lateral changes observed. Half of the participants had an increase in medial cartilage thickness at 12 M that exceeded the minimal detectable change. Standardized response mean values were moderate-to-large.
Discussion
Postoperative measures of cartilage thickness are repeatable, consistent and sensitive to change when artifact is eliminated, and a validated blinding technique is used. These results provide proof of concept for accurately measuring increases in medial knee articular cartilage after medial opening wedge HTO.







Similar content being viewed by others
References
Kraus VB, Collins JE, Hargrove D, Losina E, Nevitt M, Katz JN et al (2017) Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis 76(1):186–195
Wirth W, Hunter DJ, Nevitt MC, Sharma L, Kwoh CK, Ladel C et al (2017) Predictive and concurrent validity of cartilage thickness change as a marker of knee osteoarthritis progression: data from the osteoarthritis initiative. Osteoarthr Cartil 25(12):2063–2071
Chu CR, Sheth S, Erhart-Hledik JC, Do B, Titchenal MR, Andriacchi TP (2018) Mechanically stimulated biomarkers signal cartilage changes over 5 years consistent with disease progression in medial knee osteoarthritis patients. J Orthop Res 36(3):891–897
Le Graverand MH, Buck RJ, Wyman BT, Vignon E, Mazzuca SA, Brandt KD et al (2010) Change in regional cartilage morphology and joint space width in osteoarthritis participants versus healthy controls: a multicentre study using 3.0 Tesla MRI and Lyon–Schuss radiography. Ann Rheum Dis 69(01):155–162
Eckstein F, Guermazi A, Gold G, Duryea J, Le Graverand MH, Wirth W et al (2014) Imaging of cartilage and bone: promises and pitfalls in clinical trials of osteoarthritis. Osteoarthr Cartil 22(10):1516–1532
Lohmander LS, Hellot S, Dreher D, Krantz EF, Kruger DS, Guermazi A et al (2014) Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 66(7):1820–1831
Hargreaves BA, Worters PW, Pauly KB, Pauly JM, Koch KM, Gold GE (2011) Metal-induced artifacts in MRI. Am J Roentgenol 197:547–555
Luo J, Huang S, Gong M, Li L, Yu T, Zou X (2015) Two-level anterior cervical discectomy and fusion using self-locking stand-alone polyetheretherketone cages with two anchoring clips placed in the upper and lower vertebrae, respectively. Eur J Orthop Surg Traumatol 25(1):147–153
Huang W, Chang Z, Song R, Zhou K, Yu X (2016) Non-fusion procedure using PEEK rod systems for lumbar degenerative diseases: clinical experience with a 2-year follow-up. BMC Musculoskelet Dis 17(1):53
Hak DJ, Fader R, Baldini T, Chadayammuri VB (2017) Locking screw-plate interface stability in carbon-fibre reinforced polyetheretherketone proximal humerus plates. Int Orthop 41(9):1735–1739
Kurtz SM, Devine JN (2007) PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 28(32):4845–4869
Li CS, Vannabouathong C, Sprague S, Bhandari M (2015) The use of carbon-fiber-reinforced (CFR) PEEK material in orthopedic implants: a systematic review. Clin Med Insights Arthritis Musculoskelet Disord 8:33–45
Zimel MN, Hwang S, Riedel ER et al (2015) Carbon fiber intramedullary nails reduce artifact in postoperative advanced imaging. Skelet Radiol 44:1317–1325
Getgood A, Collins B, Slynarski K et al (2013) Short-term safety and efficacy of a novel high tibial osteotomy system: a case controlled study. Knee Surg Sports Traumatol Arthrosc 21(1):260–269
Roberson TA, Momaya AM, Adams K, Long CD, Tokish JM, Wyland DJ (2018) High tibial osteotomy performed with All-PEEK implants demonstrates similar outcomes but less hardware removal at minimum 2-year follow-up compared with metal plates. Orthop J Sports Med 6(3):2325967117749584
Cho DY, Liau WR, Lee WY, Liu JT, Chiu CL, Sheu PC (2002) Preliminary experience using a polyetheretherketone (PEEK) cage in the treatment of cervical disc disease. Neurosurgery 51(6):1343–1350
Poolman RW, Struijs PA, Krips R, Sierevelt IN, Marti RK, Farrokhyar F et al (2007) Reporting of outcomes in orthopaedic randomized trials: does blinding of outcome assessors matter? JBJS 89(3):550–558
ASTM (2001) Standard test method for evaluation of MR image artifacts from passive implants
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K et al (1986) Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum 29(8):1039–1049
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteoarthrosis. Ann Rheum Dis 16(4):494
Peterfy CG, Schneider E, Nevitt M (2008) The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthr Cartil 16(12):1433–1441
Wirth W, Nevitt M, Le Graverand MP, Benichou O, Dreher D, Davies RY et al (2010) Sensitivity to change of cartilage morphometry using coronal FLASH, sagittal DESS, and coronal MPR DESS protocols—comparative data from the Osteoarthritis Initiative (OAI). Osteoarthr Cartil 18(4):547–554
Wirth W, Eckstein F (2008) A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging 27(6):737–744
Eckstein F, Ateshian G, Burgkart R, Burstein D, Cicuttini F, Dardzinski B et al (2006) Proposal for a nomenclature for magnetic resonance imaging based measures of articular cartilage in osteoarthritis. Osteoarthr Cartil 14:974–983
Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Meas 20(1):37–46
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 2:420–428
Portney LG, Watkins MP (2009) Foundations of clinical research: applications to practice, 3rd edn. Pearson Education, Inc., New Jersey
Hunter DJ, Zhang W, Conaghan PG, Hirko K, Menashe L, Reichmann WM et al (2011) Responsiveness and reliability of MRI in knee osteoarthritis: a meta-analysis of published evidence. Osteoarthr Cartil 19(5):589–605
Birmingham TB, Moyer R, Leitch K, Chesworth B, Bryant D, Willits K et al (2017) Changes in biomechanical risk factors for knee osteoarthritis and their association with 5-year clinically important improvement after limb realignment surgery. Osteoarthr Cartil 25(12):1999–2006
Eckstein F, Yang M, Guermazi A, Roemer FW, Hudelmaier M, Picha K et al (2010) Reference values and Z-scores for subregional femorotibial cartilage thickness—results from a large population-based sample (Framingham) and comparison with the non-exposed Osteoarthritis Initiative reference cohort. Osteoarthr Cartil 18(10):1275–1283
Sharma L, Eckstein F, Song J, Guermazi A, Prasad P, Kapoor D et al (2008) Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum 58(6):1716–1726
Eckstein F, Wirth W, Hudelmaier M, Stein V, Lengfelder V, Cahue S et al (2008) Patterns of femorotibial cartilage loss in knees with neutral, varus, and valgus alignment. Arthritis Care Res 59(11):1563–1570
Wirth W, Nevitt M, Le Graverand MP, Lynch J, Maschek S, Hudelmaier M et al (2014) Lateral and medial joint space narrowing predict subsequent cartilage loss in the narrowed, but not in the non-narrowed femorotibial compartment–data from the Osteoarthritis Initiative. Osteoarthr Cartil 22(1):63–70
Moyer R, Wirth W, Eckstein F (2017) Longitudinal changes in magnetic resonance imaging-based measures of femorotibial cartilage thickness as a function of alignment and obesity: data from the osteoarthritis initiative. Arthritis Care Res 69(7):959–965
Wirth W, Larroque S, Davies RY, Nevitt M, Gimona A, Baribaud F et al (2011) Comparison of 1-year vs 2-year change in regional cartilage thickness in osteoarthritis results from 346 participants from the Osteoarthritis Initiative. Osteoarthr Cartil 19(1):74–83
Eckstein F, Mc Culloch CE, Lynch JA, Nevitt M, Kwoh CK, Maschek S et al (2012) How do short-term rates of femorotibial cartilage change compare to long-term changes? Four year follow-up data from the Osteoarthritis Initiative. Osteoarthr Cartil 20(11):1250–1257
Funding
This work was supported in part by the Canadian Institutes of Health Research (FRN-133489) (Operating Grant), the Arthritis Society (SOG-13-20) and Canada Research Chairs Program. Funding sources were not involved in the conduct of the study.
Author information
Authors and Affiliations
Contributions
RM: study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision. TB: study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision. FE: study conception and design, analysis and interpretation of data, drafting of manuscript, critical revision. WW: acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision. SM: acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision. BC: acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision. JRG: study conception and design, analysis and interpretation of data, drafting of manuscript, critical revision
Corresponding authors
Ethics declarations
Conflict of interest
Drs. Birmingham and Giffin report Grants from Canadian Institutes of Health Research and Grants from The Arthritis Society, outside of the submitted work. Dr. Birmingham also reports Grants from Canada Research Chairs Program, outside of the submitted work. Dr. Eckstein reports Grants from NIH, Grants from EU FP7-PEOPLE-2013-ITN, during the conduct of the study; personal fees from Chondrometrics GmbH, Ainring, Germany, personal fees from MerckKGaA, personal fees from Samumed, personal fees from Tissuegene, personal fees from Servier, personal fees from Roche, personal fees from Medtronic, Grants from Orthotrophix, Grants from MerckKgaA, Grants from Samumed, Grants from Tissuegene, Grants from Boston Imaging Core Lab, outside the submitted work. Drs. Wirth and Maschek report personal fees from Chondrometrics GmbH, Ainring, Germany. Drs. Moyer and Chronik have nothing to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moyer, R., Birmingham, T., Eckstein, F. et al. Validation of a novel blinding method for measuring postoperative knee articular cartilage using magnetic resonance imaging. Magn Reson Mater Phy 32, 693–702 (2019). https://doi.org/10.1007/s10334-019-00766-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-019-00766-y